
The ban on TiO2 (E171) in food

Ban on TiO2 (E171) in food – chronology and state of play
By the AVICENN team – Last added November 2025
This page compiles information about the ban on the additive E171 (titanium dioxide, partially nanoparticulate, used in particular in food as white coloring or varnish). This ban, in force in France since 2020, in Europe since August 2022, and in Switzerland since September 2022, is the result of collective work and contributions from NGOs (supported by AVICENN), scientists, media, parliamentarians and public authorities.
Please note:
- Most French manufacturers and distributors did not wait for the law to remove TiO2 nanoparticles and/or E171 from their products.
- To learn more about the risks of ingesting titanium dioxide nanoparticles, click here.
- Many voices are calling for a ban on titanium dioxide in medicines and toothpastes.
- And the E171 ban has had a ripple effect: E171 is now banned in over 36 countries, and in the United States, major companies like Mars and Tyson Foods are anticipating a ban and removing titanium dioxide from their products.
In 2025
- November 2025 : Publication by INRAE of the report entitled Ban on Titanium Dioxide as a Food additive (E171): Support for Public Policies Regulating the Use of Nanomaterials in Food (with references to AVICENN’s work)
- September 2025 : In the United States, the American agrifood group Tyson Foods has announced it will ban titanium dioxide from its products by the end of 2025 (along with high-fructose corn syrup and other ingredients such as sucralose and the antioxidants BHA/BHT).
- July 2025: Despite the ban on E171 that has been in place since 2020 in France, titanium dioxide nanoparticles have been detected in human, animal, and infant milks by French researchers from INRAE, AP-HP, Synchrotron Soleil, and CNRS.
→ When will titanium dioxide (TiO2) be banned in cosmetics and medications? - June 2025: In the United States, Mars removed the additive E171 from its famous Skittles. The announcement made by Bloomberg was widely reported by English-language media, up to India.
- April 2025 : Agave syrup sold at Carrefour is being recalled on the official RappelConso website, due to the presence of titanium dioxide not declared by the agave supplier and prohibited from being used in food:

In 2024
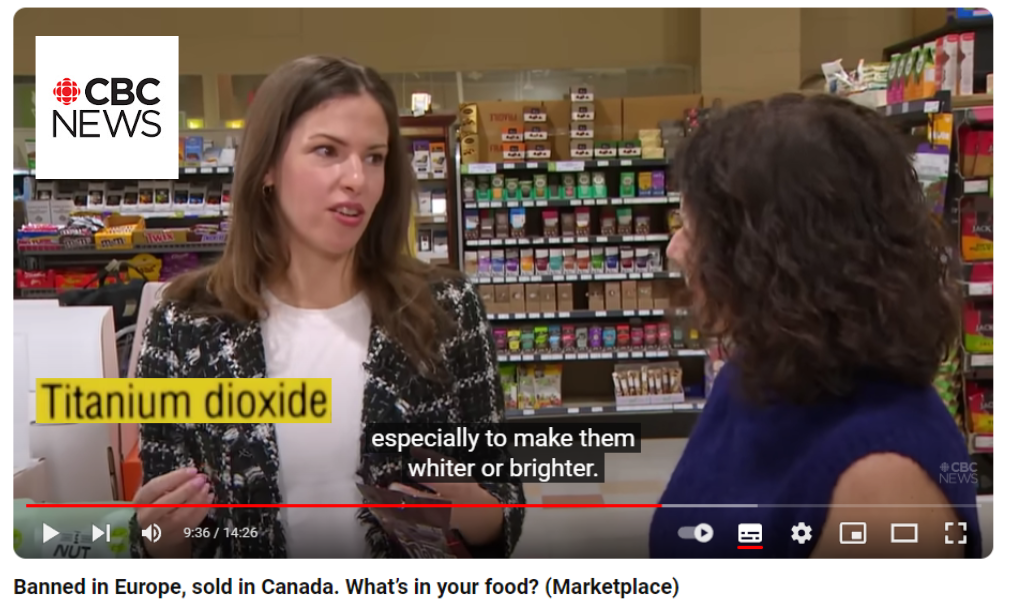
- February 2024 : In a report broadcast on CBC News, the Market Place team investigates why certain food additives are still allowed in the USA and Canada, whereas they are banned in Europe. Among the organisms that are interviewed, the European Food Safety Agency (EFSA), which ruled in 2021 that the safety of E171 could not be established (see below), and the watchdog Center for Science in the Public Interest (CSPI) which is campaigning for a ban on E171 in the USA and Canada.
In 2023
- October 2023: The Turkish Food Codex Food Additives Regulation was updated on October 13, 2023, and bans titanium dioxide in food products from April 2024.
- September 2023: A study by the French authorities, published in the journal Food Control, reports the results of controls carried out between 2018 and 2022 by the DGCCRF (fraud control) and the DGDDI (customs department) on the presence of E171 in food products sold in France: out of the 352 food products and 19 food additives analyzed, titanium was found in 152 samples (with a proportion of nanoparticles varying from 5.3 to 88.1%, the average being around 23.7 % and the median at 21.5%).
Logically, the ban on E171 in 2020 led to a substantial reduction in food products containing E171: the percentage fell from 68% in 2018 to 17% in 2021.
Products imported onto the European Union market are those where E171 is mainly – but nevertheless illegally – found: in 2022, 63% of food products containing E171 came from non-EU countries. - 1st of August 2023: The French website “Rappel Conso” publishes today a recall of frozen donuts by “The Nationals” because of the presence of titanium dioxide (E171).
- July 2023 : Ban on E171 in food comes into force in Oman
- June 2023 : Ban on E171 in food comes into force in Israel
- April 2023 : at least three food products have been recalled by French authorities since the beginning of the year, because they contained the additive E171:

- February 2023: After being banned in Yemen, Qatar and Saudi Arabia in October 2022, E171 has been withdrawn from the list of authorized additives by the Gulf Standardization Organization (GSO) in February 2023. It will be banned in Bahrain from next October.
In 2022
- December 31, 2022: The Jordan Food and Drug Administration (JFDA) bans the production and import of food products containing titanium dioxide in Jordan.
- December 28, 2022 : The results of the 2020-2021 inspections carried out by the DGCCRF on E171-containing foodstuffs on the French market show that TiO2 was still being used, despite the ban, by some bakers, distributors of foreign products, vendors of oriental pastries or artisan chocolatiers (who kept using transfer foils containing titanium dioxide in the manufacture of chocolate). The authorities issued 94 warnings, 29 administrative police measures and 9 criminal fines.
- December 23, 2022: The ban on the marketing foodstuffs containing E171 has been renewed for 2023 in France by the decree of December 23, 2022. It prevents the sale on French territory of stocks placed on the European market while the European ban implemented in August 2022 does not provide for specific withdrawal measures for these products.
- December 14, 2022: The Anses published its opinion on the risk assessment of the nanometric fraction of the food additive E171 which points out the lack of toxicological data available to perform a complete assessment of the additive E171 and recommends limiting the uses and exposures of workers and consumers to nanomaterials, “by promoting the use of safe products, free of manufactured nanomaterials, and by limiting these uses to those considered in fine as duly justified and subject to a documented demonstration of risk acceptability”.
- October 2022: Following EFSA’s opinion on E171 and SFDA circular No FS-CIR-1-V1/ 220421 of April 21, 2022, E171 is banned in Yemen, Qatar and Saudi Arabia.
- September 21, 2022: A review of the scientific literature published in the journal Nanomaterials, carried out on nearly 800 scientific articles by a research team in Portugal, concludes that a ban on titanium dioxide nanoparticles in food is justified.
- August 8, 2022: From this date, foodstuffs containing E171 will no longer be allowed to be placed on the European market. According to the European regulation 2022/63 of January 14, 2022 banning the food additive titanium dioxide (E171), those that had been previously allowed will remain so until their date of minimum durability or their use-by date.
- July 2022: In the United States, the newspapers (The Guardian, The Washington Post, Los Angeles Times, the New York Times, etc.) relay the complaint (to be considered a class action) filed by a consumer in the Federal Court for the Northern District of California against the company Mars which continues to market Skittles candies containing E171, even though the company committed to withdraw it from its products in 2016 (and that it has been withdrawn from M&M’s in France). Titanium dioxide as a food additive is currently still approved by the Food and Drug Administration (FDA).
- June 23, 2022: A Guardian article entitled “Food additive or carcinogen? The growing list of chemicals banned by EU but used in US” points out the lesser protection of Americans with respect to E171, soon to be banned in the European Union but still considered safe in the US.
- June 21, 2022: Following the EFSA opinion on E171, the European Commission has asked the Scientific Committee on Consumer Safety (SCCS) to re-evaluate the safety of TiO2 in cosmetics, with regard to its genotoxicity in case of inhalation and oral exposure. Among the types of cosmetics mentioned: lip balms, lipsticks, toothpastes, powders and sprays. The CSSC has nine months to issue its recommendations, which should therefore be finalized in March 2023.
- March 9, 2022: In Switzerland, the Federal Office of Food Safety and Veterinary Affairs (OSAV) announces a ban on the use of titanium dioxide as a food additive as of March 15, 2022, with a transitional period of six months (foodstuffs may still be produced and marketed under the old law until September 15, 2022; after this date, they may be supplied to consumers until the expiry of the best-before date).
- 18 January 2022: The European regulation 2022/63 of January 14, 2022 banning the food additive titanium dioxide (E171) has been published in the Official Journal of the EU. It will come into force on February 8, 2022 and provides that from August 8, 2022, food containing E171 can no longer be placed on the market. Those that were previously allowed may remain so until their minimum durability date or their use-by date. From 2025, this colorant could be banned in medicines. (As a reminder, France had already forbidden the use of E171 in food since January 1, 2020. No change in France, but our European neighbors will now also benefit from this protection measure).
In 2021
- 31 December 2021: The order of December 21, 2021 suspending the marketing of foodstuffs containing the additive E 171 (titanium dioxide – TiO2) was published in the Official Journal No. 304. Pending the Commission regulation to ban the use of this additive at the Community level (during 2022) and in order to ensure that no new foodstuffs containing E171 are placed on the French market, this order renews the suspension of this additive already in force since 2020 in France “banned from French food products but still present in medicines”, as pointed out by the Midi libre.
- 30 November 2021: Commission Implementing Regulation (EU) No. 2021/2090 of November 25, 2021 concerning the “refusal of authorization of titanium dioxide as a feed additive for all animal species” has been published in the Official Journal of the European Union (OJEU). It states that the authorization of titanium dioxide (E 171), as an additive in animal feed of the category “sensory additives” and of the functional group “colorants” is refused. Existing stocks and premixes containing it must be removed from the market by March 20, 2022. Feed materials and compound feeds produced with the additive or premixtures must be withdrawn from the market by 20 June 2022 at the latest.
- October 8, 2021: Member states approved a ban on titanium dioxide in food throughout the European Union. The text should come into force at the beginning of 2022. A 6-month phase-out period will then begin, at the end of which a total ban on E171 in food products will apply. This decision was voted in the Standing Committee on Foodstuffs (SCFCAH), on the proposal of the European Commission, following the scientific opinion of the European Food Safety Authority (EFSA) concluding that E171 can no longer be considered safe as a food additive (see below). This news was applauded by the NGOs that worked for this ban (including Agir pour l’Environnement, Foodwatch and WECF at the French level, BEUC, CEO and HEAL or SAFE at the European level). More info here.
- June 9, 2021: The site “Rappel Conso” publishes today an alert on Leader Price sugar-free chewing gums which contain the additive E171 banned from sale in France, because of the potential risk of colorectal cancer associated with the ingestion of titanium dioxide (partly in the form of nanoparticles).
- 31 May 2021 : Four MEPs (Maria Arena (S&D), Martin Hojsík (Renew), Mick Wallace (The Left), Anja Hazekamp (The Left)) asked the European Commission to answer the following three questions:
- When does the commission plan to release its proposal and when will the review of the proposal begin?
- What is the timetable for the discussion and adoption of the proposal, “given that it is important to introduce the ban as soon as possible in order to give priority to the health and safety of people in the European Union”?
- Does the Commission intend to restrict the additive E171 to non-food use, including medicines and cosmetics?
- 18 May 2021: At the CPVADAA working group meeting, EFSA presented its proposal for a ban on E171 in Europe to the Member States and the European Commission ; the Member States will be reconvened after the summer to vote on the timetable for the ban to come into effect – with a right of scrutiny by the European Council and Parliament afterwards, which is necessary before the ban is finally adopted.
- May 11, 2021: After France, after the European Union, it’s Switzerland’s turn to announce a ban on E171, according to Radio Télévision Suisse (RTS).
- 10 May 2021 : A few days after the announcement of the upcoming ban on E171 in Europe, three substitutes to E171 are mentioned in an article of FoodIngredients1st – with what guarantees regarding their safety?:
- “Sensient’s” Avalanche
- “White Diamond” by Doehler
- BeneoMars’ rice starches1replace Tio2 in its M&Ms recipes with rice starch.
- 6 May 2021: The European Commissioner for Health and Food Safety, Stella Kyriakides, has announced that the European Commission was going to propose a European ban on E171

→ This announcement was made just hours after the publication of the notice of the European Food Safety Agency (EFSA) concluding that this additive can no longer be considered “safe”, due to potential genotoxic effects (DNA damage). This is a clear shift in EFSA’s position, which until now had been adamant that E171 is safe – despite the numerous scientific publications that have been accumulating for several years and showing adverse effects. This turnaround confirms the relevance of the warnings voiced – for more than ten years now – by scientists and associations and taken seriously by the French authorities, who have suspended E171 since 2020. See below.
- 15 April 2021 : The new governmental website for product recall information “RappelConso” has issued three alerts concerning confectionery and chocolate containing E171, a food additive banned from sale in France because of the potential risk of colorectal cancer associated with the ingestion of titanium dioxide (partly in the form of nanoparticles):
- Harry Potter Jelly Slugs (Jelly Belly)
- Bubblegum flavored chewing gum (Bubblicious)
- White Litchi Rose chocolate bars (Klaus).
- January 26, 2021: The Arte documentary La Grande Malbouffe looks back at the suspension of E171, with some of the associative, scientific, institutional and industrial actors who played a decisive role in this issue:

In 2020
- 23 December 2020: Publication in the Official Journal of the order of December 21, 2020 “suspending the marketing of foods containing the additive E 171 (titanium dioxide – TiO2)”:

- October 22, 2020: The association Agir pour l’Environnement launches a petition “Stop titanium” asking for the extension of the ban of titanium dioxide in food (and its extension to medicines and toothpastes). In particular, because of the presence of nanoparticles.

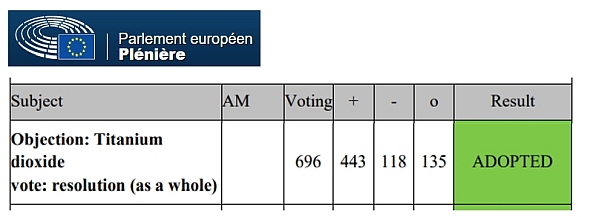
- October 8, 2020: The European Parliament, meeting in plenary session, voted, by an overwhelming majority, the objection filed by several MEPs against the European Commission’s proposal to allow E171 additives that contain up to 50% titanium dioxide nanoparticles.
- October 7, 2020: Exposure of pregnant women to titanium dioxide leads to accumulation of TiO2 nanoparticles in the placenta and contamination of the fetus. This study was conducted by scientists from INRAE, LNE, Groupe de Physique des Matériaux de Rouen, CHU de Toulouse, Université de Picardie Jules Verne and Ecole Nationale Vétérinaire de Toulouse. It confirms strong presumptions, following the publications of tests on animals. As the INRAE press release reminds us, the use of titanium dioxide in foodstuffs has been suspended in France, but it is still used in toothpastes, anti-UV screens, cosmetic creams and powders and pharmaceutical products. Hence the growing demands for its ban in cosmetics and medicines.
- September 7, 2020: The European Parliament’s Environment Committee (ENVI Committee) has rejected, by 51 votes to 11 the proposal of the European Commission to ban E171 additives that contain more than 50% of nanoparticles of titanium dioxide – which would mean allowing those that contain less than 50% of these nanoparticles. MEPs call on the Commission to apply the precautionary principle and remove E171 from the list of authorized food additives .
- May 26, 2020: The European Commission has proposed to ban E171 additives that contain more than 50% nanoparticles. France is theoretically not affected by this measure since E171 is no longer authorized on French soil since January 1, 2020 – at least in the food industry (medicines would be concerned). But the other member states have just approved this long-awaited Community framework. Their vote must still be confirmed by the European Parliament and Council this summer. Such a measure raises several questions, notably about the 50% threshold chosen – unrelated to any health consideration. Read more here.
- May 15, 2020: A review of the scientific literature by CEA researchers shows that particles of titanium dioxide (TiO2), of nanometric size and microscopic, lead to DNA damage on various cell types, including intestinal cells even at realistic low doses. Read more here.
- March 24, 2020: Research conducted in Belgium and published in 2020 showed that 12 E171 food additives, out of 15 tested, met the 2011 European definitional recommendation for the term “nanomaterial” as long as the minimum median external dimension was less than 100 nm.
- January 13, 2020: According to a CPVADAAA meeting minutes.* of December 19, 2019 by the Dutch government, the European Commission reportedly said it “respects the French measure” but has no intention of extending it to the rest of the European Union. As previously mentioned, the Commission is awaiting the next EFSA opinion due at the end of 2020 and will continue its work on the specifications concerning the particle size distribution of E171 and the limitation of heavy metals.
- January 1, 2020: The suspension of the food additive E171, composed of (nano)particles of titanium dioxide comes into effect in France. In a press release published a few days earlier, the association Agir pour l’Environnement welcomed this “historic and courageous decision” and stressed that manufacturers and distributors have already largely anticipated this suspension: there are now almost no products containing E171 in France. This is a “new proof of the uselessness of this additive” according to the association, which recalls at the same time its wish that this suspension of titanium dioxide is “extended to all products likely to be ingested” (toothpastes and medicines), in view of the danger of the additive confirmed by scientific studies compiled by AVICENN.
In 2019
- December 17, 2019: 34 Members of the European Parliament (Belgian, Croatian, Greek, Irish, Italian, Luxembourg and French) wrote to the European Commissioner for Health Stella Kyriakides asking her to ban E171 in products sold in Europe due to health risks.
- December 5, 2019: Eight French and European associations sent a joint letter to the new European Commissioner for Health, Stella Kiriakides, asking her to support France’s ban on E171 and to extend it to the rest of Europe.
- December 3, 2019: Agir pour l’Environnement has published a list of brands and retailers that have removed E171 from their products
- December 2, 2019: Following the RTS program on nanoparticles in food, the European Chemical Industry Council (CEFIC) wished to provide a “clarification” published on the website of the program that “EFSA has confirmed the absence of risk attached to the additive” [Editor’s note: a particularly biased interpretation of the opinion of EFSA whereby CEFIC does not relay the mentions relating to the uncertainties actually underlined by the European food safety agency], that “other studies conducted in accordance with OECD guidelines have not demonstrated adverse effects” and that “the Food and Environment Research Agency in the United Kingdom, the Tübitak Marmara Research Center in Turkey and the Institute for Food Safety (RIKILT) in the Netherlands have conducted a study on the oral consumption of nanometric particles and in particular titanium dioxide. This study found no significant risk from exposure to nanoparticles.”. This study is from 2015. And CEFIC does not mention the numerous studies published since then, which show alarming effects.
- November 19, 2019: Nanoparticles in food are on the menu of “A bon entendeur”, the Swiss consumer reference program, entitled tonight: “E171, E551… would you mind a few more additives?”. LNE, INRA of Toulouse, Agir pour l’Environnement and AVICENN were among the organizations surveyed in France:
- November 22, 2019: In response to MEP Eric Andrieu’s request to extend the E171 ban to all of Europe, Andriukaitis, European Commissioner for Health and Food Safety in the Juncker Commission, says that the European Food Safety Authority (EFSA) considers “the use of titanium dioxide as a food additive to be safe in the light of current scientific knowledge” and that “ongoing toxicity testing, which is expected to be completed by July 2020, will further reduce the remaining uncertainties. The Commission is considering its next steps. Read more here.
- November 4, 2019: The petition launched by the NGO SAFE (Safe Food Advocacy Europe) in partnership with Agir pour l’Environnement and ECOS to call for an EU-wide ban on E171 has surpassed 80,000 signatures.
- October 17, 2019: E171 was on the agenda of the report on nanoparticles of the program “La Quotidienne” on France 5, with Danielle Lanquetuit of VICENN and Francelyne Marano of the University Paris-Diderot. The show is available for replay here.

- September 30 – October 4, 2019: Eric Andrieu, Member of the European Parliament, has sent a written question to the European Commission in order to know whether the Member States will decide on the French suspension of E171 and whether the Commission is considering extending the latter at the European level to protect all consumers. Two days later, the MP wrote a tweet to point out this issue and recommendation supported by the European Consumers’ Organisation (BEUC) and other associations. Malik Duhaut, a Fleishman-Hillard lobbyist, replied that EFSA found that “there was nothing to question the safety of E171”. An answer “liked” by Dervla Gleeson, lobbyist for the British American Tobacco company (a connection with the fact that TiO2 is used by the tobacco industry, to whiten cigarette paper at least, and maybe also in filters?)
- September 30, 2019: The Foodwatch Association Netherlands announces, in a press release in Dutch that several brands have promised to remove E171 from their food products: Remia, Mora, Mars, Goodbite, Lindt, Haribo and A.Vogel following recommendations issued on August 21 by the Dutch Ministry of Food’s Bureau of Research and Risk Assessment (BuRO) to reduce consumer exposure to E171.
- September 26, 2019: The French suspension of E171 was still on the agenda of a CPVADAAA meeting* . Member States have again expressed their preference for a harmonized approach at European level, based on the next EFSA opinion expected in July 2020. (See the minutes of the meeting)
- September 26, 2019: The press release in which Michigan State University presents its study minimizing the effects of the additive E171 headlines the “premature” nature of the French suspension of E171. He claims that the INRA researchers (whose 2017 publication received a lot of media and government attention) did not use a control group (known as “DMH only”) in their carcinogenesis experiments, which is false, as this group was indeed part of the results. Two weeks earlier, the American study in question had been singled out by a coalition of associations, questioning its scientific rigor and independence.
- September 16, 2019: The French ban on E171 was on the agenda of an expert meeting that took place in Brussels. As in May, the vast majority of Member States have come out in favor of a harmonized measure at the European level, based on the next EFSA opinion expected in July 2020. Several associations had previously asked their government to support the French measure and encouraged the European Commission to remove E171 from the list of authorized additives, like Test Achats in Belgium. A petition in English has been launched by the NGO Safe Food Advocacy Europe (SAFE). On the other hand, manufacturers or companies using E171 are lobbying the authorities to oppose any restriction of this additive, as shown for example by the joint letter from the German food, chemical, dye and pharmaceutical industries to the German Ministry of Food, Agriculture and Consumer Protection sent shortly before the meeting.
- September 12, 2019: Several NGOs react following the publication of an American study minimizing the risks of the controversial additive E171 , a fortnight before a meeting in Brussels that examines the French ban on E171. Based on initial troubling elements surveyed by AVICENN (colon samples “obscured” in an unexplained way, near doubling of colorectal cancer markers considered “not significant” by the authors), Agir pour l’Environnement, Health and Environment Alliance (HEAL), Foodwatch, Center for International Environmental Law (CIEL), Safe Food Advocacy Europe, European Environmental Citizens’ Organisation for Standardisation (ECOS) question* the scientific rigor of the study funded by three industry associations reluctant to see E171 removed (the TiO2 Manufacturers’ Association (TDMA), the Color Manufacturers’ Association (IACM) and the Trade Association (GMA)) . * See the inter-associative press release
- August 30, 2019: The Foodwatch association reveals the presence of nanoparticles of titanium dioxide in products Dr. G. B. Oetker in Germany After France, E171 must be banned everywhere in Europe, the NGO claims.
- August 21, 2019: The Office of Research and Risk Assessment (BuRO) has released a notice in which he recommends to the Dutch Ministry of Food to discuss with manufacturers to reduce exposure to E171, to look into the presence of titanium dioxide in other products (including medicines), to advance research on the link between E171 and colorectal cancer.
- July 2019: Mentos Pure Fresh chewing gum ad ends with “No titanium dioxide!”
- 12 July 2019: The European Food Safety Authority (EFSA) has published its scientific opinion on the physicochemical characteristics of the additive E171 : Scientific opinion on the proposed amendment of the EU specifications for titanium dioxide (E 171) with respect to the inclusion of additional parameters related to its particle size distribution.
- 11 July 2019: Several of the NGOs that signed the inter-associative letter sent in early May to the European Commission were received by the latter to discuss the possibilities of extending the French suspension of E171 to the entire European Union.
- July 5, 2019: The Spanish magazine OCU-Compra Maestra revealed that E171 and E551 contained in all 8 food products tested by the Spanish consumer association “Organización de Consumidores y Usuarios” (OCU) contain nanoparticles. The proportion of E171 in the product range varies (from 27 to 76% for the 4 products containing E171, 100% for the 4 products containing E551), without mentioning [nano] on the packaging, contrary to the regulations. The consumer association OCU demands the re-evaluation of these additives.
- June 2019: Livsmedelsföretagen, the Swedish food trade federation, reports that companies in the sector have informed interested institutions that “the Commission and other Member States should firmly reject the French measure” to avoid the confusion that the French suspension would cause in terms of the internal market.
- June 2019: The Belgian magazine Test santé n°151 devotes five pages to nanomaterials; it reveals that the E171 contained in the 6 food products tested contain nanoparticles, in varying proportions (ranging from 7 to 80%), without mention [nano] on the packaging contrary to the regulations and requests the suspension of the marketing of E171 in Belgium (among other additives).
- May 29, 2019: The French suspension of E171 is endorsed all the way to the US by researchers interviewed by The Guardian who are also concerned about the health effects induced by nanoparticles in food.
- May 23, 2019: In Italy, the consumer association Altroconsumo publishes the results of tests conducted on food products, which show high levels of nanoparticles in the food additives E171, E174 (silver) and E551 (silica) but not mentioned on the label. The association asks not only the suspension of E171 but also theapplication of the precautionary principle for other nanoparticulate additives.
- May 20, 2019: Sanofi announces that it is considering substituting titanium dioxide in its drugs
- May 13, 2019 (completed May 21 and 29): French authorities presented the suspension of E171 to the European Commission and other EU member states at a CPVADAAA meeting* in Brussels. Member States have expressed a preference for a harmonized approach at European level (cf. the report of the Dutch authorities and the report of the European Commission published online on May 28). Three days earlier, EFSA had confirmed that the data provided by industry so far do not allow a proper assessment of the additive while considering that the ANSES report had not highlighted any major new findings that would overturn the conclusions of its two previous scientific opinions on the safety of E171 of 2016 and 2018. A new EFSA opinion, based on data possibly completed by then by the TiO2 manufacturers, is announced for July 2019. A vote is expected to take place later on whether to extend, repeal or modify the French measure. To be continued…
- May 9, 2019: In turn, the Léo Lagrange association – Defense of consumers (AALDC) publicly regrets the government’s “policy of small steps” regarding the suspension of E171. In particular, the limitation of its scope to food products sold in France, the late date of coming into force of the text, as well as the tolerance towards manufacturers regarding the disposal of stocks.
- May 7, 2019: The consumer association CLCV considers that “the government could have been more ambitious” regarding the suspension of E171: the decree includes, according to the association, “obvious shortcomings”, especially regarding the stocks of products, whose disposal will still be possible after 2020
- May 3, 2019: In a letter sent today, some 40 European associations asked the European Commission to extend the suspension of E171 to the entire European Union (and at the very least, not to cancel the measure in France).
- April 25, 2019: Publication in the J.O. of the Order of April 17, 2019 suspending the marketing of foods containing the additive E 171 (titanium dioxide – TiO2)
- April 19, 2019: The titanium dioxide manufacturers’ federation (TDMA) regrets France’s decision to suspend E171. The scientific argument, which is questionable, is coupled with economic considerations, undoubtedly more in line with the interests of the federation: the fear of “fragmentation and disruption of the single European market”.
- April 17, 2019: It is now official: The additive E171, which contains nanoparticles of titanium dioxide, will be banned in foodstuffs from January 1, 2020 .
- April 15, 2019: The National Health Safety Agency (ANSES) has indeed submitted its report on the risks associated with E171 to the Minister of Economy and Finance. It concludes that it does not have any new elements to remove the uncertainties on the safety of the additive E171. It reiterates its general recommendations on nanomaterials, aimed in particular at limiting the exposure of workers, consumers and the environment, by promoting safe alternatives. Bruno Le Maire had pledged to involve the associations that signed the tribune of Le Monde in December 2018 in the drafting of the decree that will enact the suspension of E171. They have reiterated their expectation that the suspension will come into effect as soon as possible (see their communiqués relayed on our twitter feed).
- April 1, 2019: The Member of the European Parliament Guillaume Balas (from the Génération.s movement) published on his site the European Commission’s response to the written question on E171 he asked in January: the European Commission “considers that there is currently no reason to apply precautionary measures with regard to the authorization of titanium dioxide as a food additive. A response that the MP considers “not up to the health challenge. In application of the precautionary principle, the Commission has the possibility to withdraw from the market a product that may pose a potential danger to the health of European citizens. It is therefore a choice on the part of the Commission to limit its action and to prefer the economic interests of companies to the health of citizens“. Guillaume Balas says he will continue his battle “to expose this hypocrisy and protect the health of Europeans”.
- March 28, 2019: The association Agir pour l’Environnement was received by the cabinet of the Minister of Economy and Finance, Bruno Le Maire to whom it requested the extension of the suspension of titanium dioxide (expected in the feed in mid-April) to all products that can be totally or partially ingested: toothpastes and medicines in particular. The representatives of the Ministry have excluded to widen the scope of the decree to products other than food, but confirmed that the decree of suspension of titanium dioxide in food will be taken in mid-April, in the wake of the publication of the opinion of the ANSES. More details here.
- March 25, 2019: In his column “War of lobbies around a titanium dioxide additive” of the Magazine de la Santé on France 5, the journalist Rudy Bancquart returned to the suspension of E171, part nano, tracing the industrial lobbying and citizen counter-lobbying.
- March 21, 2019: AVICENN has become aware of a Report of the meeting of the European Commission’s Group of Governmental Experts on Additives held on February 1. In this document, we can understand that France gives itself until April 19 to make its decision, that is to say 4 days after the expected date of return of the opinion of the ANSES on E171.
- February 6, 2019: According to the Official Journal, the Government’s report to Parliament on the measures taken concerning the import and placing on the market, free of charge or against payment, and uses by the general public, of any foodstuff containing titanium dioxide as a food additive (E 171) has been transmitted to the Committee on Economic Affairs and to the Committee on Regional Planning and Sustainable Development of the Senate.
- 21 January 2019: The ANIA, which represents the food industry in France, describes as a “regulatory Frexit” the unilateral suspension of E171 envisaged by the French government, which “would decredibilize the European authorities suggesting that Europe is lax and ineffective on health issues.” The ANIA nevertheless recognizes that the time has come to simplify recipes, with shorter lists of ingredients and the elimination of non-essential food additives. Concerning E171, the steps to eliminate or substitute it have already been taken (substitution is, however, complex, costly, and cannot be done overnight, and the alternatives must also be evaluated)
- 18 January 2019: MEP Guillaume Balas (of the Génération.s movement) calls on the European Commission to apply the precautionary principle by suspending E171 at the European level (the suspension announced by Bruno Le Maire concerns only France).
- January 11, 2019: Umpteenth rebound in the soap opera concerning the suspension of E171: Bruno Le Maire finally committed himself to sign the decree of suspension of E171 by next April 15! The 22 signatories of the article published in Le Monde in December have been invited to a meeting at Bercy at 2:30 pm in the presence of Bruno Le Maire. The Minister acknowledged mistakes in the management and communication of this file and recognized the need to implement the precautionary principle to protect public health. The associations present – including Agir pour l’Environnement, CLCV, foodwatch, France Nature Environnement, Générations Futures, Sciences citoyennes, Léo Lagrange, UFC Que Choisir, 60 millions de consommateurs – welcome this clarification from the Minister, even if they regret that this suspension will take three more months. To relive and follow on twitter.
- January 10, 2019: The deputy Delphine Batho said this afternoon on her twitter account that she has requested that the minister Bruno Le Maire, who refuses to apply the law, be summoned before the relevant committees of the National Assembly. In addition, the arbitration on the suspension of E171 would be “not yet completed” according to Agir pour l’Environnement, which met with the cabinet of François de Rugy in late afternoon. These words reported by Le Journal de l’Environnement qualify the picture: the interministerial arbitration does not seem to be over yet.
- January 9, 2019: Following the announcement of Bruno Le Maire not to suspend E171, reactions are pouring in. AVICENN relays them on the twitter account Veillenanos and will soon compile them on this website. To be continued…
- January 8, 2019: The Minister of the Economy Bruno Le Maire confirmed tonight in the program “C à vous” on France 5 that he did not intend to sign the suspension of (nano) particles of titanium dioxide in food (additive E171) for many months (or even longer: he wishes to wait for the opinion of the ANSES, and depending on this, request a new referral to the EFSA, etc.). This answer confirms the elements that we had collected during the ANSES dialogue committee on November 26, those relayed by APMnews on December 26 (which cited as a reason that the Ministry could not go against the European Commission) followed by a Europe 1 article of January 2, which stated that the department had gone so far as to say that “it is now up to the consumer to be careful”.
In 2018
- 24 December 2018: In an article published in Le Monde today, 22 organizations call on Minister Bruno Le Maire, to make effective as soon as possible the suspension of the food additive E171, composed of titanium dioxide (some of which in the form of nanoparticles). Despite a strong commitment from the government and parliamentarians, Bercy is engaging in a blockade that is deemed unacceptable by the co-signatories, who are calling for the implementation of this important public health measure without further delay. When this information was announced, the MP Matthieu Orphelin immediately reacted on twitter : “Article 53 of the #EGalim law is very clear: suspension of the additive E171 and a report to parliament before Jan 1, 2019 on the proper implementation of this decision. Let’s not go backwards”.
- November 26: In contradiction with the official position of the French authorities, the DGCCRF indicated that it did not intend at this stage to draft the decree of application of the suspension of E171, on the grounds that the evidence of “serious or immediate danger” had not yet been provided. This statement contradicts the government’s commitment in the spring, reinforced by the vote of the Parliament in the fall as part of the Food Law (this temporary ban is one of the few measures applauded by the associations to have been retained in the final version of the law). This announcement of the DGCCRF was made during the dialogue committee “nano and health” of the ANSES, in which Avicenn and several other associations participated. More information provided, upon request, to our members and associate members.
- November 8: “Now that the law has been published in the Official Journal, do you have more information on the timetable and content of the order concerning the suspension of E171 as provided for in its article 53”? This is the question that AVICENN asked again to the DGCCRF (answer pending).
- November 7, 2018: Fabrice Nesslany, from the Pasteur Institute, considers that “the usefulness (of E171) is so low, and with the doubts that still exist today (…), it is useless, so while we wait for more consolidated studies, let’s not use it” in a nano conference at the Maison de la Chimie in Paris
- November 2, 2018: Héloïse Proquin’s thesis defense on the role of E171 in the development of colorectal cancer at Maastricht University in the Netherlands: “the classification of E171 as free of toxic effects due to its insolubility and inertness is no longer valid (…); the presence of inflammation found in animal models after ingestion of E171 could aggravate inflammatory bowel disease and its adverse effects on the development of colorectal cancer. Therefore, we recommend that experiments (…) with emphasis on human testing, be conducted for further evaluation of E171 on its potential adverse effects on cancer recovery, immune system dysregulation and inflammation. This new data would provide human effects information for a full risk assessment, which could lead to a change in the use of E171 in food products: reducing the amount of nanoparticles, setting a maximum level of use in food products, more strictly limiting the types of products in which it can be used, or even suspending the product itself.”.
- November 1, 2018: The Food Law has been published in the official journal: according to its article 53…
… “The placing on the market of the additive E 171 (titanium dioxide-TiO2) and foodstuffs containing it is suspendedunder the conditions set out in the Article L. 521-17 of the Consumer Code and section 54 of the Regulation (EC) No 178/2002 of the European Parliament and of the Council of 28 January 2002 laying down the general principles and requirements of food law establishing the European Food Safety Authority and laying down procedures in matters of food safety. The Government shall submit a report to Parliament by 1 January 2019 at the latest on all measures taken concerning the importation and placing on the market, free of charge or against payment, of any foodstuff containing titanium dioxide as a food additive (E 171) and consumer uses.”
- 18 October 2018: AVICENN asks the Ministry of the Environment when the ministerial decree acting the suspension of E171 will be published via social networks (facebook & twitter)
- October 8, 2018: a few days after the announcement by Casino of its commitment to remove nanoparticles of titanium dioxide from all its products, the association Agir pour l’Environnement calls on the government to publish as soon as possible the decree allowing the suspension of the marketing and use of titanium dioxide by the end of the year. This is what Brune Poirson promised on May 18 (see below).
- 5 October 2018: AVICENN asks the DGCCRF when will be published the ministerial order acting the suspension of E171
- October 2, 2018: final vote by the National Assembly of the Food/Agriculture law (law for “the balance of commercial relations in the agricultural and food sector and a healthy, sustainable and accessible food for all”) with the article providing for the suspension of the marketing of the additive E171 as well as of foodstuffs containing it (article 11 sexdecies of Text No. 714 transmitted to the Senate on September 15, 2018, qualified as “conforming”)
- September 19, 2018: friends of the earth Germany publishes analytical results of Jacobs cappuccino powder and Wrigleys chewing gums, containing 100% silicon dioxide (E551) and 8% titanium dioxide (E171) nanoparticles respectively
- July 18, 2018: in the latest version of the Food Law resulting from the Joint Committee of July 18, 2018, Article 11 sexdecies (unamended) reads:
“The placing on the market of the additive E 171 (titanium dioxide TiO2) as well as foodstuffs containing it is suspended, under the conditions provided for in Article L. 521-17 of the Consumer Code and in Article 54 of Regulation (EC) No 178/2002 of the European Parliament and of the Council of 28 January 2002 laying down the general principles and requirements of food law, establishing the European Food Safety Authority and laying down procedures in matters of food safety
The Government shall, by January 1, 2019, submit a report to Parliament on all measures taken concerning the importation and placing on the market, free of charge or against payment, of any food containing titanium dioxide as a food additive (E 171) and consumer uses.”
- July 4, 2018: EFSA has considered that the four studies mentioned by France to request the suspension of E171 certainly pointed to concerns, but contained uncertainties, thus limiting their relevance to risk assessment. It concluded, once again, with the adage “further research is needed to reduce the level of uncertainty”
- June 29, 2018: confirmation by the Senate of the vote of the National Assembly in favor of suspending the marketing of the additive E171 as well as foodstuffs containing it: see Amendment 734 adopted as part of the “Food Law”
- June 26, 2018: the National Confectionery Union releases its charter of ethics, in which 100% of the confectioners “have committed to removing titanium dioxide from their products.“ The charter formalizes a decision made back in 2017: 90% of confectioners have already eliminated E171 by mid-2018. “Science advances, so do safety requirements. We need to go beyond regulation and anticipate consumer expectations” Florence Pradier, general secretary of the “Confiseurs de France”, said.
- May 27, 2018: vote by the National Assembly of the government’s amendment No. 2557 to suspend “the marketing of the additive E171 (titanium dioxide – TiO2) as well as foodstuffs containing it” under the Food Law
- May 18, 2018: announcement by Secretary of State Brune Poirson of a withdrawal of E171 from the market before the end of 2018
- May 16, 2018: sending of a Communiqué from 8 NGOs to Members of Parliament calling for the suspension of E171 to be brought forward as soon as possible, without waiting until 2020 (Agir pour l’environnement, le Comité pour le développement durable en santé, France nature environnement, Foodwatch, Générations cobayes, Générations futures, Réseau Environnement Santé and Women in Europe for a common future)
- April 21, 2018: opposition to the suspension of E171 from Agriculture Minister Stephane Travert, more favorable to intervention at the European level
- March-April 2018: deputies (LREM, France Insoumise) tabled amendments to the law on balance in the agricultural and food sector, aiming at the suspension of the food additive E171; other deputies (LR) tabled an amendment in the opposite direction
- February 15, 2018: France asks Europe to suspend the marketing and use of titanium dioxide as a food additive (E171) of European origin and the suspension of imports from third countries of all foods containing this additive.
- January 2018: On the basis of the tests it had carried out on products sold in France, UFC-Que Choisir lodged complaints against food manufacturers for non-compliance with the legal obligation to indicate [nano] on the packaging
In 2017
- November 2017: the fraud control (DGCCRF) confirms the presence of unlabeled nanoparticles in food, already denounced by various associations
- October 2017: during the Etats généraux de l’alimentation, Agir pour l’Environnement, France nature environnement and Générations Futures ask, with the help of a mini comic book, for a ban on E171 in all products likely to be ingested (food, but also medicines and toothpastes)
- August 31, 2017: the government asks ANSES to accelerate work on nanos in food and communicates on titanium dioxide nanoparticles in food
- August 2017: 60 Millions de consommateurs publishes new tests showing the presence of nanoparticles in food
- July 2017: Gerhard Rogler of the University of Zurich warns: “patients with intestinal barrier dysfunction, as in colitis, should abstain from foods containing titanium dioxide.”
- April 2017: In its Opinion on a request for advice on dietary exposure to titanium dioxide nanoparticles, the Anses confirms that the INRA study published in January highlights previously unidentified effects, including potential carcinogenesis promoting effects, and stresses the need to conduct the necessary studies to fully characterize the potential health effects related to the ingestion of the food additive E171.
- January 2017: The Government refers to the National Agency for Food, Environmental and Occupational Health Safety (Anses) on the conclusions of the INRA study published a few days earlier (see press release, January 20, 2017)
- January 2017: INRA researchers warn of precancerous lesions and immune problems caused by E171 in rats (cf. Food-grade TiO2 impairs intestinal and systemic immune homeostasis, initiates preneoplastic lesions and promotes aberrant crypt development in the rat colon, Bettini S et al, Scientific Reports, 7:40373, published online January 20, 2017)
In 2016
- September 2016: The authorization of the food additive E171 in force in Europe since 1969 is supported by a scientific opinion of the European Food Safety Authority (EFSA) published this month according to which the [rare] data available for food would not highlight any health problems for consumers. EFSA gives the green light to the continued use of a widespread additive, but without having conducted tests or robust studies to assess the real effects of our cumulative consumption (daily and throughout life, via different products: food, toothpaste, drugs, etc.) while Scientific publications however report adverse health effects related to the ingestion of E171.
- June 2016: The association Agir pour l’Environnement publishes first tests attesting to the presence of unlabeled nanoparticles in food in France
- April 2016: Restriction of products containing nanomaterials is included in the roadmap of the 2016 environmental conference
- April 2016: Francelyne Marano, from the University of Paris-Diderot, writes in her book Should we be afraid of nanos? : “when their addition does not correspond to a specific need other than improving the attractiveness of the product, for example in candy or chewing gum (…), [nanoparticles of titanium dioxide] should be prohibited because they do not provide any benefit”
- February 2016: AVICENN publishes the book “Nanomatériaux et risques pour la santé et l’environnement – Soyons vigilants !“
In 2015
- December 2015: AVICENN compiles and relays eleven recommendations from civil society on nanos including the banning of E171
- March 2015: MEP and anti-malnutrition activist José Bové called for a boycott of food products containing E171, holding up packages of M&M’s and Hollywood chewing gum on a TV set

In 2013
- June 2013 : AVICENN presents its Dossier Nano and Food in the framework of the ANSES nano dialogue committee
In 2010-2011
- AVICENN launches https://veillenanos.fr and informs NGOs and public authorities about nanos
In 2009
- October 2009: NGOs, including France Nature Environnement and Friends of the Earth, call for a moratorium on nanoparticles in food as part of the national public debate on nanotechnology
Other news on the topic
Upcoming Nano Agenda
- Introductory seminar on the principles of Life Cycle Assessment (LCA) and its specificities when applied to nanomaterials (metal nanoparticles, oxides, etc.), from their production to their end-of-life.
- Organizer: NaMasTE research group (Manufactured Nanomaterials, Toxicology, Ecotoxicology and Risks: Towards Controlled Development)
- Speaker: Gaetana (Tania) Quaranta, Senior Lecturer, University of Strasbourg – IPHC
- Website: https://gdr-namaste.cnrs.fr/

- Webconference for analysis laboratories, plant fertilizer manufacturers and distributors, public authorities…
- Moderated by David Krupka, nanotechnologies development manager at AFNOR Normalisation and Emilie Langlois-Bertrand, nantechnologies standardization project manager.
- In partnership with Armand Masion (CEREGE) and Patrice Charpentier (ANSES).
- This exchange will also be an opportunity to explore the creation of a national platform to identify standardization needs.
- Website: https://www.afnor.org/evenements/qualite/nanotechnologies-agriculture-cadre-pratique-responsable

- International conference on metallic nano-objects for experts working in the interdisciplinary field of metallic nanoparticles, with a particular emphasis on nanoparticle synthesis and characterization, plasmonics, optics and photonics, catalysis, biomedicine, electronics, and nanoparticle recycling
- Organizers / Partners: CNRS, Bordeaux University, Bordeaux INP, ICMCB, CRPP, CBMN, ISM
- Website: https://mno2026.sciencesconf.org
Notes and references
- 1replace Tio2 in its M&Ms recipes with rice starch



