
INRAE warns of new adverse effects from oral exposure to titanium dioxide
Published within a few days of each other, two articles from INRAE alert us to oral exposure to titanium dioxide which, although banned in food in Europe, is still very present in toothpastes and medicines.
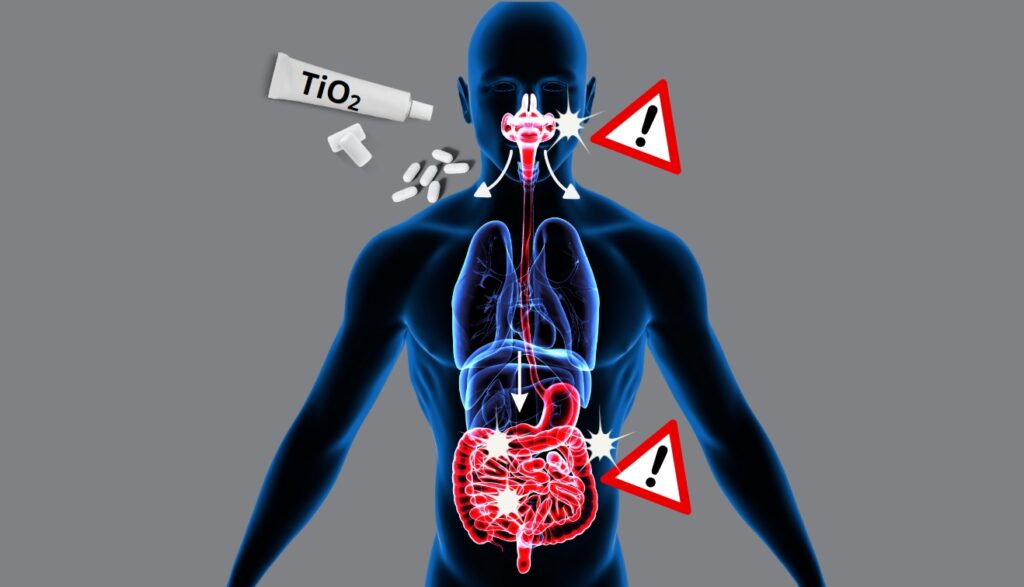
The mouth, first entry point and first target of TiO2 nanoparticles
In an article published today in the journal Nanotoxicology1See also INRAe’s press release in English, researchers from INRAE and LNE provide very important data on two aspects:
- They show that titanium dioxide nanoparticles can pass directly into the blood via the mucous membranes of the mouth;
- and they report a deterioration of the DNA of human oral cells exposed in vitro to the same TiO2 nanoparticles.
As a reminder, previous works of INRAE and other research teams had shown the penetration of nanoparticles in the body and the fetus as well as potentially carcinogenic effects in the colon.
These new research works underline the importance of taking into account the direct exposure via the oral cavity to titanium dioxide nanoparticles in human risk assessment, not only for food products, but also for toothpastes and in medicines – especially those in oro-dispersible forms (those that melt under the tongue).
If some of the TiO2 nanoparticles pass into the bloodstream as soon as they enter the mouth, what happens to those that reach the intestine?
In the intestine too, TiO2 is likely to lead to disorders promoting diabetes and obesity
In an article published a few days earlier in Environmental Pollution, INRAE researchers link the ingestion of dietary titanium dioxide (the additive E171) to certain metabolic disorders leading to diseases such as diabetes or obesity.
The researchers compiled, analyzed and synthesized studies on the effects of oral exposure to titanium dioxide on the microbiota and immune system of the gut. They compared these effects with the imbalances of the intestinal flora and intestinal inflammation observed in obese or diabetic patients. It turns out that chronic exposure to TiO2 leads to changes in the microbiota that are found in obese people, as well as metabolic disorders (such as glucose intolerance) that could promote the development of type 2 diabetes.
Because of the health issues at stake, INRAE researchers call for a better study of the still insufficiently explored effects of chronic, low-dose oral exposure to titanium dioxide nanoparticles on the intestinal microbiota. They also recommend that more attention be paid to perinatal exposure, which is particularly likely to induce significant alterations. Finally, they recommend studying TiO2 exposure in combination with other dietary inorganic (nano)particles in order to develop prevention and remediation strategies.
→ Banned in food in Europe recently, TiO2 is still authorized for the moment in toothpastes and medicines and outside Europe, it is allowed in food where its use has been exponential, particularly in North America, despite a growing number of questions in both Canada and the United States. Note: E171 was banned in Yemen, Qatar and Saudi Arabia in 2022, then removed from the list of authorized additives by the Gulf Standardization Organization (GSO ) on February 28, 2023. It will also be banned in Bahrain from next October.
Upcoming Nano Agenda

- E-learning program: awareness-raising for personnel who come into contact with nanomaterials during research, formulation, production, maintenance, cleaning, upkeep, etc., as well as safety coordinators or engineers, facility managers, heads of laboratories where nanoparticles are handled.
- Organizers: INSTN Grenoble (CEA)
- On the program:
- 1 – Introduction, definition and characteristics of nanomaterials
- 2 – Toxicity of nanomaterials: the state of knowledge
- 3 – Metrology and characterization of nanomaterials
- 4 – Prevention and protection against nanomaterials in the workplace
- 5 – Quiz: assessment of learning outcomes
- The 2-hour course can be viewed for one month from the date of registration.
- Website: https://instn.cea.fr/…risques-lies-aux-nanomateriaux…

- Scientific conference
- 23rd International conference on Advanced Nanomaterials
- From July 23 to July 25, 2025
- Website: www.advanced-nanomaterials-conference.com

- E-learning program: awareness-raising for personnel who come into contact with nanomaterials during research, formulation, production, maintenance, cleaning, upkeep, etc., as well as safety coordinators or engineers, facility managers, heads of laboratories where nanoparticles are handled.
- Organizers: INSTN Grenoble (CEA)
- On the program:
- 1 – Introduction, definition and characteristics of nanomaterials
- 2 – Toxicity of nanomaterials: the state of knowledge
- 3 – Metrology and characterization of nanomaterials
- 4 – Prevention and protection against nanomaterials in the workplace
- 5 – Quiz: assessment of learning outcomes
- The 2-hour course can be viewed for one month from the date of registration.
- Website: https://instn.cea.fr/…risques-lies-aux-nanomateriaux…
Notes and references
- 1See also INRAe’s press release in English









