
Only 300,000 tons of nanomaterials each year in France?
After more than two years delay, the two reports presenting the data collected in 2020 and 2021 via the r-nano portal for the mandatory reporting of nanomaterials have finally been published. The 2022 report is still awaited and as for the 2023 declarations, manufacturers must complete them by May 31. While 2023 coincides with the 10th anniversary of the r-nano register, many challenges still remain to be met in order for this pioneering, but still too limited, system to finally fulfill its objectives.
Figures awaited for more than two years
Better late than never: the 2020 and 2021 figures of the r-nano register have finally been published on the website of the Ministry of Ecology and on the website of the French National Health Security Agency (anses), after more than two years delay for the 2020 report1The Environmental Code requires that this data be made available to the public no later than six months after the deadline for reporting by industry, which in this case is May 1. The 2020 review, should have been published in February 2021 (the reporting period having been extended by more than four months, until August 24, 2020, due to the COVID-19 health crisis) and more than one year for the 2021 assessment2It should have been published in December 2021, with a one-month postponement granted for the declaration, by virtue of the health crisis.
The data from the 2020 report (489 pages) covers substances in the nanoparticulate state imported, manufactured or distributed in France in 2019. The 2021 report (624 pages) focuses on nanosubstances in 2020. The 2022 review of nano substances for 2021 should have been published in November 2022 but has yet to be published. It promises to be more accurate and comprehensive, thanks to the improvements made last year (see3“Towards consolidation and better use of data”). As for the 2023 declarations, manufacturers have until May 31 to complete them on the dedicated website https://r-nano.fr managed by ANSES.
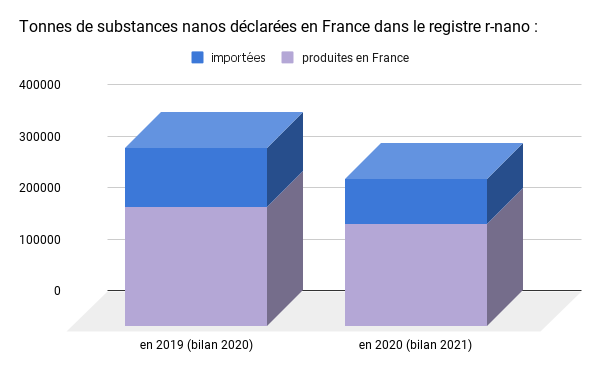
A slight decrease in the overall volume of nanos reported in 2021, driven by COVID
Data from the 2020 and 2021 reviews show a slight decrease in overall tonnage. The total quantity of substances reported (346168 tons in 20194Cf. 2020 reportcompared to 286560 tons in 20205Cf. 2021 report) thus decreased by 17% between 2019 and 2020, a decrease mainly due to the decrease in total declared production quantities6199 321 tons in 2020 versus 232,432 in 2019 attributed to the Covid-19 health crisis. Subsequent assessments will make it possible to verify whether this trend will be reversed to return to pre-crisis tonnage levels.
Little change in the main substances and uses
For 2020 and 2021, the general landscape of the nanomaterials market in France appears “rather stable compared to past years”:
- Approximately 1200 companies and laboratories located in France (more than 90% of which are distributors) have filled out a little more than 10,000 declarations in total each year.
- In 2021, 266 specific chemical categories were listed, to which must be added about 100 substances without CAS number or made up of mixtures, bringing the total number of declared substance categories to 368.
- In 2021, the 4 most produced and/or imported substances are, in order of tonnage: 1- silica; 2- carbon black; 3- calcium carbonate, which has dethroned 4- titanium dioxide, which was previously in 3rd position (in quantities of more than 10,000 tons).
- Next in 5th and 6th place are vinylidene chloride copolymer (PVDC) and polyvinyl chloride (PVC), nanopolymers used in plastics (between 1,000 and 10,000 tons).
→ Note: once again, less than 100 kg of nanosilver was reported to the r-nano registry despite a supposedly high presence in a number of products. This very low volume once again highlights the limitations of the mandatory declaration, which only covers nanosubstances produced or imported into France… but not nanosubstances contained in finished products imported into France. In other words, the nanosilver present in the “anti-odor” socks imported in France (from Asian countries for example, where nanosilver is commonly used) is not covered by the obligation of declaration and thus not present in the r-nano register, whereas it is found in French wastewater after washing. As a reminder, Anses had pointed out, as early as 20107Cf. Evaluation of the risks related to nanomaterials for the general population and the environment, Afsset (now ANSES), March 2010 the risks related to the release of silver nanoparticles during washing (even though nanosilver is known for its deleterious effects on aquatic fauna).
- In 2020 and 2021, agriculture is still the sector reporting most usage 8both in terms of the number of declarations as are the sectors of activity to which the companies reporting the most quantities produced belong.
- The 4 categories of chemicals most declared 9in terms of number of declarations are
1- plant protection products,
2- cosmetics and personal care products,
3- coatings and paints, solvents, thinners,
4- inks
→ This pole position for both agriculture and plant protection products raises again the question of the nature and quantity of nanos used in agriculture (in pesticides, fertilizers, agricultural seeds and in animal feed and veterinary drugs) and their dissemination in the environment (Anses had initiated an investigation on nanos in plant protection products in 2017, but has not communicated on the subject since).
Many challenges remain
Initiatives to consolidate and improve the use of the data contained in the r-nano registry are progressing… but at a slow pace.
A working group was set up by Anses in March 2022 to promote the consolidation and exploitation of the data from the r-nano registry. The results of this work are expected in March 2024. The ergonomics of the r-nano.fr declaration portal as well as the quality of the data provided by the declarants still need to be improved. A new IT provider, recent advances in nanometrology and the deployment of controls by the Ministry of Ecological Transition should help.
More than ten years after its creation, how much longer will it take for this register to finally fulfill its objectives, knowing that other, more ambitious projects are also necessary? Among these:
- the information made available to the public is currently limited to lists and tables of nano substances with neither the identification the products in which they are found nor the specific volumes by area of use (agriculture, paints, cosmetics, food, etc.). However, this information is necessary to better assess the risks associated with nano substances and to minimize potential unintentional exposures and environmental contamination resulting from their use.
- access to the data of the R-nano registry is still too restrictive. Five years after the High Council for Public Health (HCSP)10Cf. Bilan des connaissances relatives aux effets des nanoparticules de dioxyde de titane (TiO 2) sur la santé humaine; caractérisation de l’exposition des populations et mesures de gestion, HCSP, April 2018, a new March 2023 decree states that the data should be made available to the HCSP11Cf. Decree no. 2023-196 of March 22, 2023 concerning the availability of information obtained in application of articles L. 523-1 and L. 523-2 of the Environmental Code , in accordance with what was agreed in action 13 of the PNSE 4.
→ This (timid) first step towards broadening the accessibility of r-nano data must be followed by a broader overhaul so that all organizations and researchers working on the assessment and prevention of nano-risks can also have access to the register’s data, as AVICENN and the HCSP in particular have been requesting for years12Cf. Assessment of knowledge on the effects of titanium dioxide (TiO 2) nanoparticles on human health; characterization of population exposure and management measures, HCSP, April 2018.
- workers exposed to nanos should be automatically registered in EpiNano, the system of epidemiological monitoring of workers exposed to nanomaterials: for the past ten years, the creation of cohorts, based solely on the voluntary participation of companies, has been difficult due to the lack of company managers willing to take part.
Finally, while Belgium is also preparing to strengthen its own register and the definition of nanomaterials – although open to criticism – is becoming clearer, the prospect of a European register is emerging as an increasingly inevitable solution.
Our information sheets to go further
Upcoming Nano Agenda

- International conference on metallic nano-objects for experts working in the interdisciplinary field of metallic nanoparticles, with a particular emphasis on nanoparticle synthesis and characterization, plasmonics, optics and photonics, catalysis, biomedicine, electronics, and nanoparticle recycling
- Organizers / Partners: CNRS, Bordeaux University, Bordeaux INP, ICMCB, CRPP, CBMN, ISM
- Website: https://mno2026.sciencesconf.org
- Toxicokinetics: the fate of chemicals in the body” training course:
- the different routes of entry for toxic products
- the importance of toxicokinetics in preventing substance toxicity,
- xenobiotic absorption, distribution, metabolism and elimination
- nanoparticle toxicity
- Organizer: Association Toxicologie Chimie (ATC)
- Date: March 26, 2026
- Speaker: Nicole Proust (Research Engineer, CNRS Honorary Research Director, Palaiseau)
- Website: www.atctoxicologie.fr/…

- E-learning program: a 2-hour awareness-raising session aimed at personnel potentially exposed to nanomaterials at the workplace, heads of laboratories or facilities where nanomaterials are handled, safety coordinators or engineers.
- Organizers: INSTN Grenoble (CEA)
- On the program:
- 1 – Introduction, definition and characteristics of nanomaterials
- 2 – Toxicity of nanomaterials: the state of knowledge
- 3 – Metrology and characterization of nanomaterials
- 4 – Prevention and protection against nanomaterials in the workplace
- 5 – Quiz: assessment of learning outcomes
- The 2-hour course can be viewed for one month from the date of registration.
- Website: https://instn.cea.fr/…risques-lies-aux-nanomateriaux…
Notes and references
- 1The Environmental Code requires that this data be made available to the public no later than six months after the deadline for reporting by industry, which in this case is May 1. The 2020 review, should have been published in February 2021 (the reporting period having been extended by more than four months, until August 24, 2020, due to the COVID-19 health crisis)
- 2It should have been published in December 2021, with a one-month postponement granted for the declaration, by virtue of the health crisis
- 3“Towards consolidation and better use of data”
- 4Cf. 2020 report
- 5Cf. 2021 report
- 6199 321 tons in 2020 versus 232,432 in 2019
- 7Cf. Evaluation of the risks related to nanomaterials for the general population and the environment, Afsset (now ANSES), March 2010
- 8both in terms of the number of declarations
- 9in terms of number of declarations
- 10
- 11
- 12



