Titanium dioxide (TiO2) nanoparticles

By the AVICENN team – Last modification March 2020
Titanium dioxide (TiO2) nanoparticles
Why and how is TiO2 used?
The properties of titanium dioxide
Titanium dioxide has been used for a long time on a non-nano scale as a white pigment (combined with other colorants, it can also be used to create a range of colors) in many products (paints, medicines, toothpaste, make-up, food (until 2020), etc.).
In paints for example, the “pigmentary” particles of titanium dioxide are mostly between 200 and 350 nm to obtain an intense white color. Above 350 nm, the texture is no longer uniform, but “granular”.
With the progress of metrology techniques and tools, scientists have highlighted the fact that nanoparticles are contained in the pigmentary titanium dioxide (including ones used in the food additive E 171)1See in particular the references cited by Bettini S and Houdeau E, Oral exposure to titanium dioxide (TiO2) nanoparticles: from crossing the oral and intestinal epithelium to the fate and effects in the body, Biology Today, September 2014 :
– Titanium Dioxide Nanoparticles in Food and Personal Care Products, Weir A. et al., About. Sci. Technol., 46 (4), 2242-2250, 2012 (36% of particles with size <100 nm)
– Characterization of titanium dioxide nanoparticles in food products: analytical methods to define nanoparticles, Peters RJ et al, J Agric Food Chem, 62(27), 6285-6293, 2014
– Characterization of foodgrade titanium dioxide: the presence of nanosized particles, Yang Y et al, Environ Sci Technol, 48, 6391-6400, 2014 (17-35% particles with size <100 nm)
– Characterization and preliminary toxicity assay of nano-titanium dioxide additive in sugar-coated chewing gum, Chen XX et al, Small, 9, 1765-1774, 2013 (28-40% of particles with size <100 nm)
Also to be consulted
– Detection and Characterization of SiO2 and TiO2 Nanostructures in Dietary Supplements, Lim JH et al, J Agric Food Chem, 1; 63(12), 3144-52, April 2015, even though not necessarily all intentionally produced on this scale by the manufacturers.
Questions have therefore arisen for some time on the safety of these particles contained in many food products2See our page What are the risks associated with (nano)titanium dioxide particles (nano TiO2), leading to the suspension of E171 in early 2020. New properties that appear at the nanometric scale have been discovered over the last fifteen years making TiO₂ nanoparticles suitable for the following industrial use:
- as a brightening agent: TiO₂ nanoparticles can be used to give shine to products (food, drugs, makeup, etc.), …
- as a UV barrier, in sun creams, food packaging, clothing, …
- as an antibacterial agent3 See for example:
– Nanostructured TiO2 anatase-rutile-carbon solid coating with visible light antimicrobial activity, Krumdieck SP et al, Scientific Reports, 9: 1883, 2019
– Subsurface treatment of TiO2 nanoparticles for limestone: Prolonged surface photocatalytic biocidal activities, Veltri S et al, Building and Environment, 149: 655-661, February 2019
– Antibacterial surfaces prepared by electrospray coating of photocatalytic nanoparticles, Chemical Engineering Journal, 334: 1108-1118, February 2018 in deodorants, toothbrushes, textiles, etc… - as a self-cleaning coating4See for example
Self-cleaning materials, UVC lamps, robots… How tech will make cleaning easier, RMC, March 2023: at less than 200 nm in diameter, TiO₂ particles cause the oydation (decomposition) of organic pollutants by photocatalysis (under the effect of light); deposited on surfaces made hydrophilic, they therefore enable self-cleaning (provided that photocatalysis is complete, which is not always the case – with then negative consequences) - as a flame retardant in certain textiles
- as a coating with improved scratch resistance
In which products?
Titanium dioxide TiO₂ nanoparticles are used in many industries and products5See in particular:
– Aide au repérage des nanomatériaux en entreprise, INRS, ED 6174, June 2014
– Use of nanometric titanium dioxide – Special case of the construction industry, INRS, Hygiène et Sécurité au travail, Note documentaire 2367, 4th quarter 2012:
- in the medical and pharmaceutical sector: in medicines as a colorant, in vaccines as an excipient
- in the food sector:
- in food, beverages food, beverages and packaging as a colorant (E171) (banned in France since 2020)
- in packaging (cardboard, plastic) and plastic films, as an anti-UV barrier or as an antibacterial agent
- in the cosmetics sector:
- in makeup (mascara, nail polish, eye shadow, foundation, lipstick, skin care creams, hair coloring and bleaching) or toothpaste as a colorant (CI77891)
- in sunscreens as an anti-UV barrier
- in deodorants as an antibacterial agent
- in small care appliances, as an antibacterial agent: hairbrushes, electric shavers, toothbrushes, hair dryers, curling irons, etc.
- in the textile sector, as an antibacterial agent (for medical staff gowns, fabrics used in operating theatres), as a flame retardant (firemen’s suits) and also as a UV barrier in clothing (for beach T-shirts for example)
- in inks as a colorant (in toner printers for example), paints6According to FIPEC, titanium dioxide provides ” whiteness, opacity, gloss, stability and durability of exterior colors. By the density that it brings, it improves the texture, and allows to limit the number of layers during the application. It allows an almost infinite variety of colors”: source : http://www.fipec.org/index.php/afei/actualites-communiques-de-presse/13-sipev (last accessed December 2018), stains and varnishes as a colorant (to obtain a pearly hue7Pfaff, G. and P. Reynders, Angle-Dependent Optical Effects Deriving from Submicron Structures of Films and Pigments. Chemical Reviews, 1999. 99(7): p. 1963-1982 and Braun, J.H., A. Baidins, and R.E. Marganski, TiO2 pigment technology: a review. Progress in Organic Coatings, 1992. 20(2): p. 105-138 (cited in Le Trequesser Q, Synthesis of titanium dioxide nanoparticles of controlled morphologies: localization, quantification and toxicological aspects from the cell to the multicellular organism, thesis, Material chemistry, University of Bordeaux, 2014) as an antibacterial, self-cleaning or scratch-resistant agent
- in the automotive sector, in catalytic converters as a depolluting agent
- in the construction sector and on roads: interior or exterior coatings of buildings and materials (concrete, cement, walls, steel, stone, glass, tiles, asphalt and roads)
- in furniture and interior equipment (air purifiers) as a depollutant
- in extraction units on oil fields as a depollutant
- …
What are the quantities on the market?
Since 2014, between 10,000 and 100,000 tons of titanium dioxide nanoparticles have been declared each year as having been produced and/or imported into France8Cf. The R-Nano register – The annual declaration of “substances in the nanoparticulate state” in France, mandatory since 2013, veillenanos.fr as part of the mandatory declaration of substances in the nanoparticulate state that populates the r-nano register.
This “more or less 90,000 tons” range illustrates the weight given by the public authorities to industrial and commercial secrecy which prevents us from having a good understanding of the quantities and uses of these particles, in spite of the increasingly pressing requests for information from a growing number of stakeholders.
In France, the Thann plant in the Haut-Rhin is one of the few remaining TiO2 production sites after the closure of the Tioxide pigment TiO2 manufacturing plant in Calais (Huntsman Group)9Cf. Tioxide closes its Calais plant, L’Usine nouvelle, March 20, 2017. It belongs to the Cristal Group (Saudi Arabia), the world’s second largest producer of titanium dioxide after DuPont.
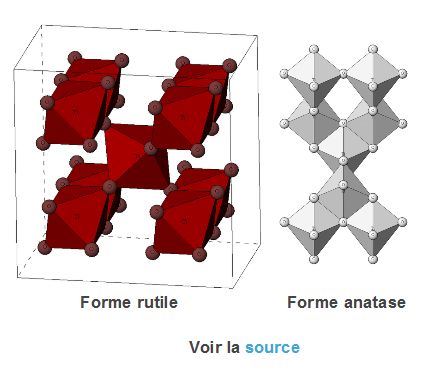
A wide variety of TiO2 nanoparticles
There is a wide variety of TiO₂ nanoparticle types, including differences in crystal form (anatase, light reactive, useful for pollution control applications/rutile, which absorbs UV), size distribution, morphology and coating:
- in foodstuffs (E171), toothpastes and medicines, they are mostly in the anatase form (sometimes associated with the rutile form in very small proportions) and without coating
- In cosmetics (Cl 77891) and sun creams, they are mostly in rutile form (or anatase/rutile mixture) and coated with a layer of silica or alumina to prevent the formation of free radicals (which cause skin aging). However, chlorine present in swimming pools can degrade this coating, and when in contact with water and under the effect of light, the nanoTiO2 can release free radicals, responsible for the aging of the skin and the emergence of cancers10In 2012, researchers in Cincinnati, USA, showed that chlorine from swimming pools can degrade the aluminum hydroxide coating that surrounds the titanium dioxide (TiO2) nanoparticles embedded in some sunscreens (here Neutrogena SPF 30). In contact with water and under the effect of light, the core of the nanomaterial, nanoTiO2, can then release free radicals, responsible for skin aging and the appearance of cancers.
– Cf. Depletion of the protective aluminum hydroxide coating in TiO2-based sunscreens by swimming pool water ingredients, Chemical Engineering Journal, 191: 95-103, May 2012
– See also this more recent article: UV filters interaction in the chlorinated swimming pool, a new challenge for urbanization, a need for community scale investigations, Sharifan H et al, Environ Res, 148:273-276, July 2016.
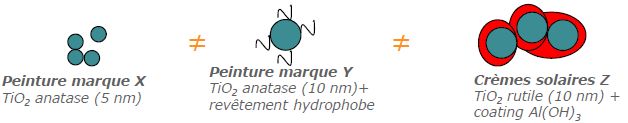
- in paints and cements (CI Pigment White 6), they are mainly in anatase form (or anatase/rutile mixture) and without coating (to allow the photocatalysis reaction aimed at destroying the dirt/pollutants)
- for depolluting / antibacterial uses, TiO₂ is not coated
Associated risks and the beginnings of specific regulations
What are the associated risks?
Nanoparticles of titanium dioxide are released into the environment, with undesirable consequences for ecosystems and humans (exposed workers but also consumers and the general public).
In view of the harmful health effects likely to be caused by nanoTiO2:
- the French Health Safety Agency (ANSES) recommended in 2014 that titanium dioxide nanoparticles be classified as hazardous substances so that measures could be put in place to restrict their use or even ban their use in certain consumer applications
- At the European level, the classification of titanium dioxide (TiO2) as a category 2 carcinogen by inhalation was registered in the Official Journal of the European Union in February 2020.
The additive E171, food titanium dioxide, banned in France and Europe
In France, the Food Law published in the Official Journal on November 1, 2018 states that “the marketing of the additive E 171 (titanium dioxide-TiO2) as well as foodstuffs containing it is suspended” (Article 53). This measure came into force on January 1, 2020, before being generalized at the European level in 2022, after a long process and collective work to which many stakeholders contributed: NGOs (supported by Avicenn), scientists, media, parliamentarians and government members.
Towards a ban of TiO2 in some cosmetics and drugs in France?
After food, several associations asked for the extension of the ban of titanium dioxide to cosmetics likely to be ingested and to medicines.
Brands are now promoting TiO2-free drug coatings.
The toothpaste.infoconso.org website of Agir pour l’Environnement was created to allow consumers to quickly identify toothpastes with or without titanium dioxide (but it has not been updated since).
“If it’s white, it’s not nano”? Not so simple…
Below the 200 nm threshold, titanium dioxide particles gradually lose their white appearance and become more and more transparent as they decrease in size.
They also continue to absorb ultraviolet rays below this threshold, which is why cosmetic brands use them to make sunscreens both more effective and transparent while being less viscous and visible. The pictures on the left show the gradation from white to transparent on two types of skins according to the dispersion of the primary particles.
The higher the proportion of small titanium dioxide particles, the more transparent the product becomes.

However, this does not mean that white titanium dioxide does not contain nanoparticles, i.e., particles with one size smaller than 100 nm, despite claims to the contrary by TiO₂ manufacturers concerned that the white colorant could be associated with a nanomaterial11See in particular:
– Additional information regarding titanium dioxide and E171 – Titanium Dioxide Manufacturers Association (TDMA) news release, March 23, 2015
– Statement in response to recent articles regarding removal of food grade TiO2 from food formulations – Press release from the International Association of Color Manufacturers (IACM), 25 March 015 (NB: in the USA, what is called “food grade TiO2” corresponds to the food grade titanium dioxide that is known as E171 in Europe) (and then be subject to the obligation of declaration to the national agency of sanitary security in France or even to a labeling obligation depending on the sectors).
In French:
- Haut Conseil de la Santé Publique (HCSP), “Le dioxyde de titane (TiO2) : caractéristiques physiques” ; “Production, utilisation, transport et stockage du TiO2” in Assessment of knowledge on the effects of titanium dioxide (TiO2) nanoparticles on human health; characterization of population exposure and management measures, April 2018 (made public in June 2018)
- Titanium dioxide in 10 points, Ministry of Ecological Transition and Solidarity, May 2018
- Titanium dioxide, Wikipedia
- production imports and exports in France in 2017 for all titanium dioxide
- Use of nanometric titanium dioxide – Special case of the construction industry, INRS, Hygiène et Sécurité au travail, Note documentaire 2367, 4th quarter 2012
In English:
- https://tdma.info: site of the TiO2 manufacturers : the Titanium Dioxide Manufacturers Association (TDMA)
- Application of Titanium Dioxide, Edited by Magdalena Janus, InTech, July 2017 (open access)
- Exposure assessment of the food additive titanium dioxide (E 171) based on use levels provided by the industry, RIVM, March 2016
- About Titanium Dioxide, Titanium Dioxide Manufacturers Association (TDMA), July 2013
Any questions or comments? This information sheet compiled by AVICENN is intended to be completed and updated. Please feel free to contribute.
Other news on the topic
Upcoming Nano Agenda
- Introductory seminar on the principles of Life Cycle Assessment (LCA) and its specificities when applied to nanomaterials (metal nanoparticles, oxides, etc.), from their production to their end-of-life.
- Organizer: NaMasTE research group (Manufactured Nanomaterials, Toxicology, Ecotoxicology and Risks: Towards Controlled Development)
- Speaker: Gaetana (Tania) Quaranta, Senior Lecturer, University of Strasbourg – IPHC
- Website: https://gdr-namaste.cnrs.fr/

- Webconference for analysis laboratories, plant fertilizer manufacturers and distributors, public authorities…
- Moderated by David Krupka, nanotechnologies development manager at AFNOR Normalisation and Emilie Langlois-Bertrand, nantechnologies standardization project manager.
- In partnership with Armand Masion (CEREGE) and Patrice Charpentier (ANSES).
- This exchange will also be an opportunity to explore the creation of a national platform to identify standardization needs.
- Website: https://www.afnor.org/evenements/qualite/nanotechnologies-agriculture-cadre-pratique-responsable

- International conference on metallic nano-objects for experts working in the interdisciplinary field of metallic nanoparticles, with a particular emphasis on nanoparticle synthesis and characterization, plasmonics, optics and photonics, catalysis, biomedicine, electronics, and nanoparticle recycling
- Organizers / Partners: CNRS, Bordeaux University, Bordeaux INP, ICMCB, CRPP, CBMN, ISM
- Website: https://mno2026.sciencesconf.org
File initially put on line in June 2016
Notes and references
- 1See in particular the references cited by Bettini S and Houdeau E, Oral exposure to titanium dioxide (TiO2) nanoparticles: from crossing the oral and intestinal epithelium to the fate and effects in the body, Biology Today, September 2014 :
– Titanium Dioxide Nanoparticles in Food and Personal Care Products, Weir A. et al., About. Sci. Technol., 46 (4), 2242-2250, 2012 (36% of particles with size <100 nm)
– Characterization of titanium dioxide nanoparticles in food products: analytical methods to define nanoparticles, Peters RJ et al, J Agric Food Chem, 62(27), 6285-6293, 2014
– Characterization of foodgrade titanium dioxide: the presence of nanosized particles, Yang Y et al, Environ Sci Technol, 48, 6391-6400, 2014 (17-35% particles with size <100 nm)
– Characterization and preliminary toxicity assay of nano-titanium dioxide additive in sugar-coated chewing gum, Chen XX et al, Small, 9, 1765-1774, 2013 (28-40% of particles with size <100 nm)
Also to be consulted
– Detection and Characterization of SiO2 and TiO2 Nanostructures in Dietary Supplements, Lim JH et al, J Agric Food Chem, 1; 63(12), 3144-52, April 2015 - 2See our page What are the risks associated with (nano)titanium dioxide particles (nano TiO2), leading to the suspension of E171 in early 2020
- 3See for example:
– Nanostructured TiO2 anatase-rutile-carbon solid coating with visible light antimicrobial activity, Krumdieck SP et al, Scientific Reports, 9: 1883, 2019
– Subsurface treatment of TiO2 nanoparticles for limestone: Prolonged surface photocatalytic biocidal activities, Veltri S et al, Building and Environment, 149: 655-661, February 2019
– Antibacterial surfaces prepared by electrospray coating of photocatalytic nanoparticles, Chemical Engineering Journal, 334: 1108-1118, February 2018 - 4See for example
Self-cleaning materials, UVC lamps, robots… How tech will make cleaning easier, RMC, March 2023 - 5See in particular:
– Aide au repérage des nanomatériaux en entreprise, INRS, ED 6174, June 2014
– Use of nanometric titanium dioxide – Special case of the construction industry, INRS, Hygiène et Sécurité au travail, Note documentaire 2367, 4th quarter 2012 - 6According to FIPEC, titanium dioxide provides ” whiteness, opacity, gloss, stability and durability of exterior colors. By the density that it brings, it improves the texture, and allows to limit the number of layers during the application. It allows an almost infinite variety of colors”: source : http://www.fipec.org/index.php/afei/actualites-communiques-de-presse/13-sipev (last accessed December 2018),
- 7Pfaff, G. and P. Reynders, Angle-Dependent Optical Effects Deriving from Submicron Structures of Films and Pigments. Chemical Reviews, 1999. 99(7): p. 1963-1982 and Braun, J.H., A. Baidins, and R.E. Marganski, TiO2 pigment technology: a review. Progress in Organic Coatings, 1992. 20(2): p. 105-138 (cited in Le Trequesser Q, Synthesis of titanium dioxide nanoparticles of controlled morphologies: localization, quantification and toxicological aspects from the cell to the multicellular organism, thesis, Material chemistry, University of Bordeaux, 2014
- 8
- 9Cf. Tioxide closes its Calais plant, L’Usine nouvelle, March 20, 2017
- 10In 2012, researchers in Cincinnati, USA, showed that chlorine from swimming pools can degrade the aluminum hydroxide coating that surrounds the titanium dioxide (TiO2) nanoparticles embedded in some sunscreens (here Neutrogena SPF 30). In contact with water and under the effect of light, the core of the nanomaterial, nanoTiO2, can then release free radicals, responsible for skin aging and the appearance of cancers.
– Cf. Depletion of the protective aluminum hydroxide coating in TiO2-based sunscreens by swimming pool water ingredients, Chemical Engineering Journal, 191: 95-103, May 2012
– See also this more recent article: UV filters interaction in the chlorinated swimming pool, a new challenge for urbanization, a need for community scale investigations, Sharifan H et al, Environ Res, 148:273-276, July 2016 - 11See in particular:
– Additional information regarding titanium dioxide and E171 – Titanium Dioxide Manufacturers Association (TDMA) news release, March 23, 2015
– Statement in response to recent articles regarding removal of food grade TiO2 from food formulations – Press release from the International Association of Color Manufacturers (IACM), 25 March 015 (NB: in the USA, what is called “food grade TiO2” corresponds to the food grade titanium dioxide that is known as E171 in Europe)