
Risks associated with titanium dioxide nanoparticles

Risks associated with titanium dioxide nanoparticles
By the AVICENN team – Last modification in March 2025
Very strong presumptions of risk
Even in non-nanoscale form, inhaled TiO₂ is possibly carcinogenic
In 2006 the International Agency for Research on Cancer (IARC) classified titanium dioxide (TiO₂) as a possible human carcinogen (class 2 B)1Carcinogenic Hazards from Inhaled Carbon Black, Titanium Dioxide, and Talc not Containing Asbestos or Asbestiform Fibers: Recent Evaluations by an IARC Monographs Working Group, Baan RA, Inhalation Toxicology, 2007, 19(1): 213-228. It should be noted that this classification has been challenged on the grounds that the studies cited were conducted on rats, whose respiratory system is different from that of humans – and this is true for all sizes: the nanoscale is therefore concerned, just as non-nanoscale TiO₂. The studies that were considered for this classification involved TiO₂ in powder form. The presumption of risk by inhalation first concerns potentially exposed workers.
Thirteen years later, at the end of a long struggle between industrialists and the French and European health authorities2 As early as September 2014, the “Board of Appeal” of the European Chemicals Agency (ECHA) had been seized by nine manufacturers of titanium dioxide (Tioxide Europe / Huntsman, Cinkarna, Cristal Pigment, Du Pont, Evonik, Kronos, Precheza, Sachtleben Chemie GmbH and Tronox Pigments) who refused to provide data requested by ECHA as part of the risk assessment associated with titanium dioxide (including its nanoforms) provided for under REACH. In March 2017, they had won their case on the grounds that the term “nanoform” was not defined in Reach. Cf. Decision of the board of appeal of the European Chemicals Agency, March 2017., the classification of TiO₂ as a category 2 carcinogen by inhalation was adopted by the European Commission on October 4, 2019 and published in February 20203Cf. COMMISSION REGULATION (EU) 2020/217 of 4 October 2019 amending, for the purposes of its adaptation to technical and scientific progress, Regulation (EC) No 1272/2008 of the European Parliament and of the Council on classification, labelling and packaging of substances and mixtures and correcting that Regulation published in the Official Journal of the European Union on 18 February 2020 and Corrigendum to Commission Delegated Regulation (EU) 2020/217 of 4 October 2019 amending, for the purposes of its adaptation to technical and scientific progress, Regulation (EC) No 1272/2008 of the European Parliament and of the Council on classification, labelling and packaging of substances and mixtures and correcting that Regulation OJEU, February 25, 2020. This is due to the retention (biopersistence) in the lungs and the low solubility of titanium dioxide.
The classification of TiO₂ became effective on October 1, 2021. When used in the form of a powder containing 1% or more of particles with an aerodynamic diameter of 10 μm or less, it is It is necessary to inform users of the precautionary measures that should be taken to minimize the risk to human health. This can be, for example, with the following statement on liquid mixtures: “Caution. Hazardous respirable droplets may be formed when spraying. Do not breathe aerosols or mists” or, for solid mixtures: “Caution. Hazardous respirable dust may be formed during use. Do not breathe this dust”. The European Chemicals Agency (ECHA) has published a guide to help companies implement the now mandatory measures to inform and protect workers4Cf. Guide on the classification and labelling of titanium dioxide, ECHA, September 2021.
However, the Titanium Dioxide Manufacturers’ Association (TDMA) announced that on May 13, 2020, some of its members had filed an appeal with the European Union General Court against the harmonized classification of TiO₂, which they sought to have annulled5See in particular:
– Legal action against the classification of titanium dioxide, TDMA, June 4, 2020
– Action brought on 13 May 2020 – Brillux and Daw v Commission (Case T-288/20), General Court of the EU. The TDMA indicated that it and its members “will work to find a way to implement the regulation from this date, despite the uncertainties of the classification.”
On November 23, 2022, the European Union General Court ruled in their favor, overturning the classification6Cf. Titanium dioxide professionals (temporarily) obtain the annulment of the classification of TiO₂ as an inhalation carcinogen, VeilleNanos, 23 November 2022. However, in February 2023, the French government, and then the European Commission, filed an appeal to contest this cancellation: the classification will therefore remain in force until the end of the procedure at least, expected to last one to two years7Cf. TiO2 classification stays, VeilleNanos, 22 February 2023. In November 2023, the ONG HEAL denounced industry interference in the TiO2 classification process and called for a review of the regulatory framework for chemicals in Europe8Cf. How industry can launder a health-risking substance, Social Europe, 8 novembre 2023.
At the nano scale, the risks could be even greater
A recent publication established the presence of titanium dioxide (TiO₂) nanoparticles in the liver and spleen of 15 humans (so, no longer just lab rats). In half of the cases, the levels were above what is considered safe for the liver9Cf. Detection of titanium particles in human liver and spleen and possible health implications, Heringa MB et al, Particle and Fibre Toxicology, 15:15, 2018.
For several years, there has been a mounting number of scientific publications on the risks associated with titanium dioxide nanoparticles.
See in particular:
– In French :
- Les actions de l’Anses sur le dioxyde de titane, Anses, 18 octobre 2024
- Note d’appui scientifique et technique (AST) relatif à l’analyse des données fournies dans le cadre de l’évaluation du dioxyde de titane (TiO2) dans les produits cosmétiques, Anses, 1er octobre 2024
- Empa, Les nanoparticules : Risque pour les embryons dans le ventre de leur mère, 6 juin 2024
- (…)
- INRA, Food additive E171: the first results of oral exposure to titanium dioxide nanoparticles, press release, January 20, 2017 and E171: a hazard identified in rats, a risk to be assessed in humans, INRA Sciences & Impacts press release, February 1, 2017
- Evaluation of the effects of exposure to TiO₂ nanoparticles on the adult and vulnerable brain, abstract in French of the thesis (in English) of Clémence Disdier, Université Paris-Saclay, April 2016
- “Mesure des effets toxicologiques de nano-oxydes métalliques sur cellules humaines in vitro”, by Grall R et al., in Participant’s file prepared for the Restitution du Programme national de recherche environnement santé travail (PNREST), October 2015
- Le Trequesser Q, Synthesis of titanium dioxide nanoparticles of controlled morphologies: localization, quantification and toxicological aspects from the cell to the multicellular organism, thesis, Material chemistry, University of Bordeaux, 2014
- Nicolle-Mir L, Review of toxicological data on titanium dioxide nanoparticles, Environment, Risks and Health, 13, 180-181, 2014 (synthesis in French of the article listed below)
- Armand L et al, TiO₂ nanoparticles: a hidden enemy, Biofutur, 32/347, 42-45, October 2013.
- INRS, Fiche toxicologie Dioxyde de titane, FT 291, 2013 edition
- Committee for Sustainable Development in Health (C2DS), Nanoparticulate titanium dioxide, Emerging risk?, November 2011
- Vivagora, CoExNano, Nanosilver and titanium dioxides in coatings: State of knowledge, uncertainties and controversies, November 2010
– In English:
- TiO2 nanoparticle-induced lung carcinogenicity, Wolf S et al., Nanotoxicology, August 2024
- Nanoparticules : Risk for babies in the womb, Empa, 6 June 2024
- (…)
- Comparative effects of TiO₂ and ZnO nanoparticles on growth and ultrastructure of ovarian antral follicles, Santacruz-Marques R et al, Reproductive Toxicology, 96: 399-412, September 2020
- TiO₂ genotoxicity: an update of the results published over the last six years Carrière M, Arnal ME, Douki T, Mutation Research/Genetic Toxicology and Environmental Mutagenesis, May 15, 2020: this review of the scientific literature by CEA researchers shows that nanosized and microscopic titanium dioxide (TiO₂) particles cause DNA damage in various cell types, lung and intestinal, even at low, realistic doses.
- Toxic lobbying: the titanium dioxide label debate continues, CEO, 2019
- Effects of Titanium Dioxide Nanoparticles Exposure on Human Health-a Review, Baranowska-Wójcik E et al, Biological Trace Element Research, 1-12, 2019 : “TiO2 NPs can induce inflammation due to oxidative stress. They can also have a genotoxic effect leading to, among others, apoptosis or chromosomal instability. (…) Regular supply of TiO₂ NPs at small doses can affect the intestinal mucosa, the brain, the heart and other internal organs, which can lead to an increased risk of developing many diseases, tumours or progress of existing cancer processes.”
- Subchronic exposure to titanium dioxide nanoparticles modifies cardiac structure and performance in spontaneously hypertensive rats, Rossi S et al, Particle and Fibre Toxicology, 16:25, 2019
- Effect of gestational age on maternofetal vascular function following single maternal engineered nanoparticle exposure, Fournier SB et al, Cardiovascular toxicology, 1-13, 2019: In rats, exposure to titanium dioxide nanoparticles in early gestation has a significant impact on the fetal circulatory system. Later exposure affects the growth of the fetus.
- NanoEHS, the database of scientific publications on nanotechnology risks, maintained by the International Council on Nanotechnology (ICON) does not seem to be working anymore (2016)
- Mitochondrial dysfunction and loss of glutamate uptake in primary astrocytes exposed to titanium dioxide nanoparticles, Wilson CL et al, Nanoscale, 7, 18477-18488, 2015: see press release: Researchers show modest levels of nanoparticle may harm brain cells, University of Nebraska-Lincoln, 15 December 2015
- Tissue biodistribution of intravenously administered titanium dioxide nanoparticles revealed blood-brain barrier clearance and brain inflammation in rat, Particle and Fibre Toxicology, Disdier et al, 12:27, September 2015
- Toxicity of TiO₂ nanoparticle to denitrifying strain CFY1 and the impact on microbial community structures in activated sludge, Li D et al, Chemosphere, 2015
- Nanosized TiO₂ is internalized by dorsal root ganglion cells and causes damage via apoptosis, Erriquez J, Nanomedicine, 11(6):1309-19, August 2015
- Titanium dioxide nanoparticles: some aspects of toxicity/focus on the development, Rollerova E, et al, Endocr Regul, 49(2): 97-112, 2015
- Environmental hazard of selected TiO₂ nanomaterials under consideration of relevant exposure scenarios, Anne J. Wyrwoll et al, study for the German Ministry of the Environment, October 2014 (see also abstract:Sunshine may increase nanoparticle toxicity, according to German agency, Chemical Watch, 21 October 2014)
- Tissue distribution and elimination after oral and intravenous administration of different titanium dioxide nanoparticles in rats, Geraets L et al, Particle and Fibre Toxicology, 11:30, 2014
- Titanium dioxide nanoparticle impact and translocation through ex vivo, in vivo and in vitro gut epithelia, Brun E et al, Particle and Fibre Toxicology, 11:13, 2014
- Stakeholders’ Response to Titanium Dioxide Manufacturers association’s letter on Titanium dioxide, EEB, BEUC, ANEC, CIEL, BUND, ETUC, ECOS, WECF, CEO, Friends of the Earth Europe, September 2013
- Titanium dioxide nanoparticles: a review of current toxicological data, Shi H et al, Particle and Fibre Toxicology, 10(15), April 2013
- Nanoparticles in Food – with a focus on the toxicity of titanium dioxide, C. Rydström Lundin, Uppsala University and the Swedish National Food Agency, 2012
- DNA damage and alterations in expression of DNA damage responsive genes induced by TiO₂ nanoparticles in human hepatoma HepG2 cells, Petkovic, J et al, Nanotoxicology, 5, 341-353, 2011
- Aqueous synthesis and concentration-dependent dermal toxicity of TiO₂ nanoparticles in wistar rats, Unnithan, J et al, Biol. Trace Elem. Res., 143, 1682-1694, 2011
- Silica and titanium dioxide nanoparticles cause pregnancy complications in mice, Yamashita, K et al, Nat. Nanotechnol., 6, 321-328, 2011
In 2014, the French Health Security Agency (ANSES) had recommended a classification of titanium dioxide nanoparticles (among others) as hazardous substances so that measures to restrict or even ban the use of certain consumer product applications could be put in place. This recommendation was included in Action No. 72 of the 3rd National Environmental Health Plan (PNSE 3) (2015-2019) and in Action 1.13 of the Occupational Health Plan (PST 3) (2016-2019).
The impact of the crystalline form on titanium dioxide toxicity remains to be refined, but it would appear that for the same surface area, anatase forms induce less inflammation than rutile forms and that the inflammatory and acute phase response is greater and more persistent for TiO₂ tubes10Cf. Effects of physicochemical properties of TiO₂ nanomaterials for pulmonary inflammation, acute phase response and alveolar proteinosis in intratracheally exposed mice, Danielsen PH et al., Toxicology and Applied Pharmacology, 386(1), January 2020; these findings qualify those of a 2018 study whose results leaned the other way toward a greater harm of anatases to the immune system when inhaled: cf. The crystal structure of titanium dioxide nanoparticles influences immune activity in vitro and in vivo, Vandebriel RJ et al, Particle and Fibre Toxicology, 15:9, 2018.
Finally, it is now scientifically proven that titanium dioxide (nano)particles, combined with other contaminants (PCBs, pesticides, etc.) can cause “cocktail effects” more harmful than the effects of these substances taken separately
In 2021, titanium dioxide nanoparticles have been identified as one of the four most risky categories of nanoparticles by a team from University College Dublin11Cf. A semiquantitative risk ranking of potential human exposure to engineered nanoparticles (ENPs) in Europe, Li, Y and Cummins, E, Science of the Total Environment, 778, July 2021.
According to a literature review by researchers from the National Institute of Occupational Health of Norway published in 202412Systematic review of mechanistic evidence for TiO2 nanoparticle-induced lung carcinogenicity, Wolf S et al., Nanotoxicology, August 2024, TiO2 nanoparticles might possess the ability to induce chronic inflammation and oxidative stress – but it was challenging to compare the findings in the studies due to the wide variety of TiO2 nanoparticles differing in their physicochemical characteristics, formulation, exposure scenarios/test systems, and experimental protocols. Given the limited number of high-quality and high-reliability studies identified within this review, there is a lack of good enough mechanistic evidence for TiO2 nanoparticles lung carcinogenicity. Future toxicology/carcinogenicity research must consider including positive controls, endotoxin testing (where necessary), statistical power analysis, and relevant biological endpoints, to improve the study quality and provide reliable data for evaluating TiO2 nanoparticles-induced lung carcinogenicity..
→ In addition to the toxicity and ecotoxicity associated with TiO₂ nanoparticles per se, there is the issue of the production and toxicity of compounds from the photocatalytic reaction: researchers have found that the decomposition of volatile organic compounds (VOCs) by TiO₂ nanoparticles incorporated in coatings or paints is not always complete and that it can generate other harmful molecules (acetone, acetaldehyde, formaldehyde in particular)13See for example:
– ADEME, Indoor air purification by photocatalysis, Technical notice, September 2020
– Gandolfo A et al, Unexpectedly High Levels of Organic Compounds Released by Indoor Photocatalytic Paints, About. Sci. Technol., 52, 19, 11328-11337, 2018
– Indoor Air Quality Observatory, Photocatalytic scrubbing – Opportunity or threat to indoor air quality, IAQO Bulletin No. 4, June 2012.
– Vivagora, CoExNano, Nanosilver and titanium dioxides in coatings: State of knowledge, uncertainties and controversies, November 2010.
Workers are the first to be exposed
Exposed workers14Cf. Prasath, S et al., Profiling Nano-Titanium Dioxide Use in Singapore: a Survey of Practices, Quantities, and Workers, Academia Materials Science, 2(1), 2025 or Exposure to nanoscale titanium dioxide in construction and public works, Bertrand Honnert, Archives des Maladies Professionnelles et de l’Environnement, INRS, May 2018 (particularly in the chemical, construction15Bonetta et al., DNA damage in workers exposed to pigment grade titanium dioxide (TiO2) and association with biomarkers of oxidative stress and inflammation, Environnemental Toxicology and Pharmacology, Volume 105, November 2023, cosmetics, textile, or food industries) should be the subject of nano-specific awareness training, protection, and monitoring.
Efforts are being made in this direction:
- Starting in 2014, data on titanium dioxide nanoparticles collected as part of the French mandatory declaration (r-nano) were communicated to the Institut national de veille sanitaire (InVS) / Santé publique France as part of the Epinano project for monitoring cohorts of workers exposed to nanomaterials. However, the system is experiencing implementation difficulties.
- In 2018, the High Council on Public Health (HCSP) called for the protection of workers and populations in the vicinity of industrial sites producing or handling nano-TiO₂, and published various practical recommendations for public authorities and industrialists16Cf. Protecting workers and people in the vicinity of titanium dioxide nanoparticle production or handling sites, High Council for Public Health, June 25, 2018.
- In early April 2019, Anses published a recommendation for a chronic inhalation toxicological reference value (TRV) for the nanoparticulate form of titanium dioxide P25 of 0.12 µg/m3 (close to that recommended by INERIS in 2016). This is the first TRV developed for a nanomaterial in France. Anses will investigate the feasibility of extending this TRV to other forms of TiO₂-NP. Based on this reference value, health risk assessments will be conducted as part of the management of industrial facilities and sites in France.
- In a December 2020 report made public in March 2021, Anses unveiled its recommendations for occupational exposure limit values (OELs) to strengthen risk prevention for workers exposed to TiO₂ nanoparticles by inhalation: 0.80 µg/m3 and a pragmatic 15-min TLV of 4 µg/m3.
- In early 2022, INRS published an updated version of the toxicological data sheet on titanium dioxide with information and recommendations on micro- and nanoscale forms of TiO2 uses, physical properties, chemical properties, occupational exposure limit values (OELs), occupational exposure assessment methods, toxicokinetics – metabolism, toxicity and genotoxicity, carcinogenic effects, reproductive effects, regulations and recommendations.
The risks for the environment are also worrying
Finally, the dissemination of manufactured nanoparticles in the environment, especially those of titanium dioxide, can be a source of toxicity for terrestrial and aquatic ecosystems17See for example:
In French :
– Doc’ en clip – the risk associated with nanoparticles in sunscreens (video), Riccardo Catalano, Aix-Marseille University, October 14, 2019
– Dynamics, reactivity and ecotoxicity of metal oxide nanoparticles in soils: impact on the functions and diversity of microbial communities, thesis by Marie Simonin (Microbial Ecology / UMR CNRS 5557 Claude Bernard University – Lyon 1), defended in October 2015
– Nano or not: the TiO₂ is toxic for the environment, The Cosmetics Observatory, October (summary in French of the report“Environmental hazard of selected TiO₂ nanomaterials under consideration of relevant exposure scenarios“, Umwelt bundesamt, October 2014).
In English:
– Proteomics reveals multiple effects of titanium dioxide and silver nanoparticles in the metabolism of turbot, Scophthalmus maximus, Araújo MJ et al, Chemosphere, 2022
– Zinc oxide, titanium dioxide and C60 fullerene nanoparticles, alone and in mixture, differently affect biomarker responses and proteome in the clam Ruditapes philippinarum, Marisa I et al, Science of the Total Environment, 838 (2), September 2022
– Toxicity of titanium nano-oxide nanoparticles (TiO2) on the pacific oyster, Crassostrea gigas: immunity and antioxidant defence, Arash Javanshir Khoei and Kiadokht Rezaei, Toxin Reviews, 41, 2022
– Lethal and sub-lethal effects of nanosized titanium dioxide particles on Hydropsyche exocellata Dufour, 1841, Torres-Garcia D et al, Aquatic Insects – International Journal of Freshwater Entomology, 41(1), 2020
– Silver and titanium nanomaterials present in wastewater have toxic effects on crustaceans and fish cells, Norwegian Institute for Water Research (NIVA), November 2019
– Evaluation of the effects of titanium dioxide and aluminum oxide nanoparticles through tarsal contact exposure in the model insect Oncopeltus fasciatus, López-Muñoz D. et al, Science of The Total Environment, 666: 759-765, May 2019
– Titanium dioxide nanoparticles impaired both photochemical and non-photochemical phases of photosynthesis in wheat, Dias MC, Protoplasma, 256(1): 69-78, January 2019
– Transfer and Ecotoxicity of Titanium Dioxide Nanoparticles in the Terrestrial and Aquatic Ecosystems: A Microcosm Study, Vijayaraj V et al, Environmental Science and Technology, 52(21): 12757-12764, October 2018
– Toxicological impact of TiO₂ nanoparticles on Eudrilus euginiae, Priyanka KP et al, IET Nanobiotechnology, 12 (5):579, August 2018
– Ecotoxicological Effects of Transformed Silver and Titanium Dioxide Nanoparticles in the Effluent from a Lab-Scale Wastewater Treatment System, Georgantzopoulou A et al., About. Sci. Technol., 52, 16, 9431-9441, 2018
– Environmental exposure to TiO₂ nanomaterials incorporated in building material, Bossa N et al, Environmental Pollution, January 2017
– Titanium dioxide nanoparticles strongly impact soil microbial function by affecting archaeal nitrifiers, Simonin M et al, Scientific Reports, 6, 2016
– Ecotoxicity of engineered TiO₂ nanoparticles to saltwater organisms: An overview, D. Minetto, G. Libralato, A. Volpi Ghirardini, Environment International, May 2014 – hence the need for environmental monitoring.
Different evaluations
In the context of REACH
The assessment of the risks associated with titanium dioxide (including its nanoforms), provided for under REACH, was to be carried out by ANSES since 2012-2013, but it has been hampered by the refusal of industry to provide the data necessary for this assessment18See the paragraph “From 2007 to 2017, ten years of arm wrestling over nanomaterials regulation (or lack thereof) under REACH” on our information sheet How are nanomaterials regulated under REACH? and the page Anses’ actions on titanium dioxyde (in French) on Anses website, 18 october 2024.
In food
The food additive E171 consists of TiO₂ particles (some of which are in nano form). It has been banned since 2020 in France, and since 2022 in Europe (after EFSA acknowledged in May 2021 that its genotoxicity can not be excluded19Cf. Titanium dioxide: E171 no longer considered safe when used as a food additive, EFSA, 6 mai 2021).
Many publications report deleterious health effects related to the ingestion of TiO₂ nanoparticles: risks for the liver, ovaries and testicles in humans, immune problems and precancerous lesions in the colon in rats, disruptions of the intestinal microbiota, inflammation and alterations of the intestinal barrier in animals as well as in humans, adverse effects on the offspring in rodents…
At the end of 2022, Anses published its opinion on the risk assessment of the nanometric fraction of the food additive E171 which points out the lack of toxicological data available to perform a complete assessment of the additive E171 and recommends limiting the uses and exposures of workers and consumers to nanomaterials, “by promoting the use of safe products, free of manufactured nanomaterials, and by limiting these uses to those considered in fine as duly justified and subject to a documented demonstration of risk acceptability”.
In November 2023, the Joint FAO/WHO Expert Committee on Food Additives (JECFA) evaluated E171 and assigned it an “ADI not specified”. These conclusions are not yet detailed, and a fuller report is due to be published shortly20Cf: WHO Technical Report Series (TSR 1051).
In cosmetics
→ in make-ups and beauty products:
The safety of titanium dioxide nanoparticles in make-ups and beauty products (CI77891) is not as well established as previously thought21See in particular Les nanoparticules de dioxyde de titane, leur place dans l’industrie cosmétique et ses dangers, Laura Daragnes, Thèse pour l’obtention du diplôme d’Etat de docteur en pharmacie, sous la direction de Isabelle Bestel, Université de Bordeaux, September 2018 and several associations are calling for a ban on TiO2 in cosmetic products likely to be ingested (toothpastes, balms and lipsticks in particular).
In its Scientific Advice on Titanium dioxide (TiO2) 1661/23 published in May 2024, the European Scientific Committee on Consumer safety (SCCS) considers that the available evidence, provided by industry for 44 pigmentary et 40 nano TiO2 grades, is not sufficient to exclude the genotoxicity potential of almost all of the types of TiO2 grades22(82 our of 84) used in oral cosmetic products and thus, that more experimental data are needed to exclude the genotoxicity potential of the grades of TiO2 (both pigmentary and nano) used in oral cosmetic products, except for two nano grades23(RM09 and RM11, for which the provided genotoxicity data indicate no genotoxicity concern).
The SCCS also adds that, considering that oral mucosal cells are prone to the uptake of nanoparticles (including TiO2 nanoparticles) and that some oral products containing TiO2 nanoparticles such as toothpastes, will be used every day and potentially more than once a day, further investigations are needed to exclude the risk to the consumer from longterm repeated exposures of the oral mucosa to TiO2 nanoparticles.
→ See also the Scientific and Technical Support NOTE on the analysis of the data provided in the context of the assessment of titanium dioxide (TiO2) in cosmetic products published by Anses in September 2024.
→ in sunscreens:
In sun creams, TiO₂ nanoparticles have been evaluated by the Scientific Committee on Consumer Safety (SCCS), which has approved their use as UV blockers, with a maximum concentration of 25% allowed (spray applications are not allowed)24CSAS issued an initial opinion on July 23, 2013, revised in 2014: Scientific Committee on Consumer Safety SCCS OPINION ON Titanium Dioxide (nano form) COLIPA No. S75, SCCS, April 2014 and completed September 2014: Scientific Opinion for clarification of the meaning of the term “sprayable applications/products” for the nano forms of Carbon Black CI 77266, Titanium Oxide and Zinc Oxide, SCCS, September 2014 (published June 2015)). The nano form of titanium dioxide particles is listed since August 2016 in Annex VI of the Cosmetics Regulation25Cf. Our sheet Framing of nanomaterials in cosmetics, veillenanos.fr . They are used mainly in rutile form (or anatase/rutile mixture) and often coated with a layer of silica or alumina to prevent the formation of free radicals (which cause skin aging).
However, several problems have been identified:
- Chlorine in swimming pools can degrade this coating and when in contact with water and under the effect of light, TiO₂ can then release free radicals, responsible for skin aging and the appearance of cancers26In 2012, researchers in Cincinnati, USA, showed that chlorine from swimming pools can degrade the aluminum hydroxide coating that surrounds titanium dioxide (TiO₂) nanoparticles embedded in some sunscreens (here Neutrogena SPF 30). In contact with water and under the effect of light, the core of the nanomaterial, the nano TiO₂ can then release free radicals, which are responsible for skin aging and the development of cancers
– Cf. Depletion of the protective aluminum hydroxide coating in TiO2-based sunscreens by swimming pool water ingredients, Chemical Engineering Journal, 191: 95-103, May 2012
– See also this more recent article: UV filters interaction in the chlorinated swimming pool, a new challenge for urbanization, a need for community scale investigations, Sharifan H et al, Environ Res, 148:273-276, July 2016. - Research in Spain and France estimated that the quantities of titanium dioxide nanoparticles released during the summer on Mediterranean beaches and showed a significant increase in the concentration of hydrogen peroxide, a toxic molecule for phytoplankton which is the basic food of marine animals27In 2014, Spanish researchers estimated that tourist activity on a Mediterranean beach during a summer day can release about 4 kg of titanium dioxide nanoparticles into the water, resulting in an increase of 270 nM/day in the concentration of hydrogen peroxide (a molecule with toxic potential, particularly for phytoplankton, which is the basic food of marine animals). Cf. Nanos UV screens: a danger for marine life, L’Observatoire des Cosmétiques, September 5, 2014
– In 2017, researchers from CEREGE in France, on the other hand, measured the concentration of titanium in the water of three beaches in Marseille and estimated the weight of TiO₂ released in the two summer months for one small beach at 54 kilograms per day. See :
– Scientists find titanium dioxide from sunscreen is polluting beaches Scientists find titanium dioxide from sunscreen is polluting beaches, presentation by Labille J., Goldschmidt Conference, August 2018
– Estimation and minimization of the risk associated with TiO2 nanoparticles used in sunscreens, presentation by Labille J, “Nano and cosmetics” technical day organized by LNE, 29 March 2018
– Pollution of coastal waters by UV absorbers from sunscreens, generated by summer activities, Labille J, OHM Littoral project, 2017. - Dermatologists from the Bichat and Rothschild Hospitals have, for the first time, observed at the Sun Synchrotron the presence of titanium dioxide (TiO₂) nanoparticles along the hair follicles of a patient suffering from fibrosing frontal alopecia (hair loss at the top of the forehead). The patient had been using TiO₂-containing sunscreens daily for the past 15 years28See in particular:
– Identification of titanium dioxide on the hair shaft of patients with and without frontal fibrosing alopecia: a pilot study of 20 patients, CT Thompson, ZQ Chen , A Kolivras, A Tosti, British Journal of Dermatology, 2019
– Titanium dioxide nanoparticles and frontal fibrosing alopecia: cause or consequence?, O. Aerts, A. Bracke, A. Goossens, V. Meuleman, J. Lambert, Journal of The European Academy of Dermatology and Venereology, June 2018
– Sunscreen, nanoparticles and frontal alopecia, Synchrotron sun, February 2018
– Detection of titanium nanoparticles in the hair shafts of a patient with frontal fibrosing alopecia, Brunet-Possenti F, Deschamps L, Colboc H, et al, Eur Acad Dermatol Venereol, 2018.. - Researchers in the Czech Republic tested a sunscreen containing nanoparticles of titanium dioxide and found that it does NOT prevent skin cancer (although it prevents the skin from turning red, it does not protect it from oxidative stress caused by UV light)29Cf. NanoTiO2 Sunscreen Does Not Prevent Systemic Oxidative Stress Caused by UV Radiation and a Minor Amount of NanoTiO2 is Absorbed in Humans, Pelclova D et al, Nanomaterials, 9(6), 888, 2019.
In March 2019, due to these risks and uncertainties around the safety of titanium dioxide nanoparticles, Cosmébio30Titanium dioxide in organic cosmetics, Cosmebio, March 2019 recommended to its members to remove titanium dioxide from their products or to replace it with an alternative when it exists.
Potential adverse effects of titanium dioxide nanoparticles in sunscreens continue to be studied31See in particular:
– Sunscreen: FDA regulation, and environmental and health impact, Shanthi Narla, Henry W. Lim, Photochemical & Photobiological Sciences, 2020
– Titanium Dioxide and Zinc Oxide Nanoparticles in Sunscreens: A Review of Toxicological Data, Vujovic M, Kostic E, Journal of Cosmetic Science, September 2019
– Fueling a Hot Debate on the Application of TiO2 Nanoparticles in Sunscreen, Sharma S et al, Materials, July 2019 and to generate controversies32See for example:
– Sunscreen products for children – Too many substances of concern, Agir pour l’Environnement and WECF France, July 2020
– Titanium dioxide nanoparticles, their place in the cosmetic industry and its dangers, Laura Daragnes, Thesis for the obtention of the State Diploma of Doctor of Pharmacy, under the direction of Isabelle Bestel, University of Bordeaux, September 2018
– Nanoparticles – Attention, elles se cachent partout, Que Choisir, Mensuel n° 566, February 2018.
Please note: Titanium dioxide nanoparticles are not expressly authorized for uses other than UV filtering. However, they are found in toothpastes, shower gels, etc. for which UV filtering is not necessary…
In toys
In June 2023, the European Scientific Committee on Health, Environmental and Emerging Risks (SCHEER) published an Opinion on the safety of titanium dioxide in toys in which it states that :
- regarding inhalation exposure: if an ultrafine (e.g nanometric) fraction is assumed to be present, safe use is NOT indicated for the use of casting kits, chalk and powder paints; only white colour pencils can be used with no or negligible risk by children of different age groups independent whether an ultrafine fraction is present in the TiO2 preparation.
- regarding oral exposure, safe use is only indicated when the absence of an ultrafine fraction in the TiO2 pigments can be demonstrated by an appropriate methodology.
SCHEER states that “in studies on TiO2 in nanosize, the results (mainly in vitro studies) show higher probability of positive response than in studies on microsize or with sizes slightly above 100 nm. It is possible that a probability of a genotoxic effect diminishes as the size of TiO2 increases, and the observed positive effects can depend on the presence of a nanofraction”.
In medicine
TiO2 is present as a colorant in many drugs (look for E171 in the information sheet). However, in 2022, the European Commission has laid the groundwork for a potential ban that could come into effect from 2025. Emphasizing that “it is crucial that the pharmaceutical industry makes every effort to accelerate research and development of alternative solutions to replace titanium dioxide (E171) in medicines”33See our fact sheet“Towards the suspension of titanium dioxide in cosmetics and medicines” for more information..
A paper published on January 28, 2019, in Nature Nanotechnology shows that titanium dioxide, silica, and gold nanoparticles can induce changes in the endothelium and thus leakage of tumor cells, leading to metastasis. According to Frédéric Lagarce, professor of biopharmacy and hospital practitioner in Angers,
“What is interesting / original is to show a potential risk of nanotechnologies in the treatment of tumors while these technologies are often presented as the answer to improve the performance of anticancer drugs. It is now necessary to verify whether these endothelial modifications are also found with polymeric or lipidic nanoparticles, which are much more commonly used to encapsulate active ingredients and target tumors. If this were unfortunately the case, the whole strategy of nanomedicine (very cancer-oriented) would be called into question“
Read more here.
In paints and building
Recent research has shown that the promises surrounding the depollution of paints containing TiO₂ nanoparticles are not delivered and that the benefit/risk balance is far from being conclusive34See for example:
– Evaluation of the Performance and Durability of Self-Cleaning Treatments Based on TiO2 Nanoparticles Applied to Cement-Based Renders and Boards, Fregni, A, Venturi, L, Franzoni, E, Coatings, 13, 990, 2023
– Photocatalytic TiO2-Based Coatings for Mortars on Facades: A Review of Efficiency, Durability, and Sustainability, Bersch, JD et al, Buildings, 13, 186, 2023
– Paints to purify ambient air, CEA Liten, November 2020.
Prior to these results, the “Release_NanoTox” project (ANSES funding 2015-2018) had attempted to provide new knowledge regarding the potential impact of nano-objects from nanocomposite materials under stress of use, on brain functions. The scientific teams had developed an experimental bench to achieve a realistic exposure using TiO₂ nanoparticles from the sanding of nanoadditive materials35The Centre Scientifique et Technique du Bâtiment (CSTB) and the LNE (MONA Platform) participated in the aeraulic characterization phase of this bench and in the physicochemical characterization of the nano-objects emitted in the exposure chamber. Then ANSES and the CarMeN laboratory were involved for the inhalation exposure and in vivo analysis showing cerebral morphofunctional alterations of the mice during the exposure36See in particular: In vivo evaluation of the potential neurotoxicity of aerosols released from mechanical stress of nano-TiO2 additived paints in mice chronically exposed by inhalation, Maxinay S et al., J. Phys: Conf. Ser, 838 012025, 2017
Research Activity Report 2016, LNE, 2016.
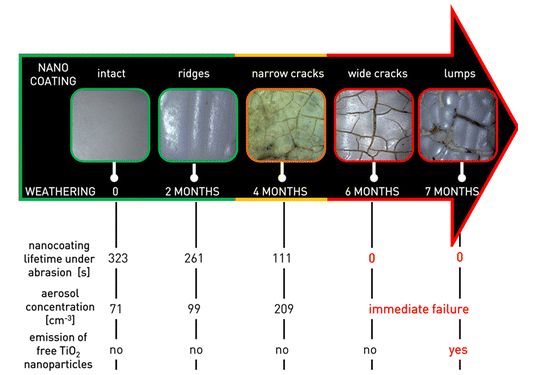
A European research project called NanoHouse conducted between 2010 and 2013 had observed the life-cycle of titanium dioxide nanoparticles contained in paints and coatings used inside and outside the home. The work estimated the release rate of nanoparticles to be only 1 to 2% – and in the form of agglomerates37Research into the safety of nanoparticles – No nano-dust danger from façade paintEMPA, January 13, 2014; subsidized to the tune of 2.4 million euros by the European Commission, out of an overall budget of 3.1 million euros, the NanoHouse project ran from January 2010 to June 2013, with French partners the CEA et ISTerre.. Other studies are much less reassuring: a study by INERIS and the University of Compiègne published in early 2015 showed that a commercially available titanium dioxide nano-coating, once applied to a building facade, can deteriorate under the effect of sunlight and rain. As a result, titanium particles are released into the air within a few months – and in the form of free particles (more dangerous than when agglomerated with each other or with residues of other materials)38Cf. Emission of titanium dioxide nanoparticles from building materials to the environment by wear and weather, Shandilya, N et al, Environmental Science & Technology, 49(4): 2163-2170, 2015; a lay summary is freely available here : Nanocoating on buildings releases potentially toxic particles to the air, “Science for Environment Policy,” European Commission, May 28, 2015. It is therefore advisable to minimize the use of nanocoatings under these conditions.
What environmental monitoring?
In June 2020, the French High Council for Public Health (HCSP) published a report regarding the metrological monitoring in the environment of titanium dioxide nanoparticles (TiO₂) finalized in October 201939Cf. Interim report – elements for environmental metrological monitoring of titanium dioxide (TiO₂) nanoparticles and feasibility review, HCSP, October 2019 (publication June 2020). For the HCSP, it is conceivable to carry out measurements of the concentration levels of TiO₂ nanoparticles in the air around industrial sites. In the absence of a single operational method to simultaneously measure particle number/mass and perform chemical analysis of nanoparticulate TiO₂, HCSP considers that different measurement approaches and methods should be considered, depending on the specifics of each situation.
Since 2019, the Tronox (ex-Cristal) plant in the Haut-Rhin is required to conduct environmental monitoring of the impacts of its titanium dioxide particle emissions, including in nanometric forms, with two measurement campaigns each year40Cf. Arrêté portant prescriptions complémentaires à la société CRISTAL France SAS à Thann, Préfecture du Haut-Rhin, 3 August 2018 ; see also Estimation of average annual concentrations in the air around an industrial site producing substances in the nanoparticulate state – Cristal site – Thann, Titanium dioxide production unit, INERIS, October 2017. In October 2020, the DREAL of the Haut-Rhin indicated to Avicenn41Cf. Letter from the DREAL of the Haut-Rhin in response to Avicenn’s request , 2 October 2020 that no isolated nanoscale TiO₂ particles were detected during the last campaign with results known at the time (June 2019). Two types of elements were observed: a small minority of agglomerates larger than 250 nm composed of nanoparticles and a large majority of spheroids larger than 100 nm and some larger than 400 nm. The 2019 concentrations were down from the exploratory campaigns conducted in 2013, 2016, and 2018 and the February 2020 concentrations in levels near the limit of quantification. Results from subsequent campaigns in the summer of 2020 were not yet available.
Caution: large agglomerates should not necessarily be considered less toxic than small agglomerates. In early 2020, the results of research conducted in Belgium were published showing that large agglomerates of TiO₂ nanoparticles do not appear to be less active than small agglomerates42Cf. Agglomeration of titanium dioxide nanoparticles increases toxicological responses in vitro and in vivo, Murugadoss S et al, Particle and Fibre Toxicology, 17(10), 2020.
Restrictions in Switzerland
In 2008, the Grand Council of the Republic and Canton of Geneva advised against the use of nanoparticulate TiO₂ on public construction sites as well as in the buildings of private companies43Health: straight into the wall… self-cleaning, Alternative Santé, January 6, 2016 and Report M 1741-A of the State Council to the Grand Council of Geneva, 2008. It was based in particular on the study carried out by the Cantonal Service of Industrial Toxicology and Protection against Indoor Pollution which considered “irresponsible to use such a product before even researching the known hazards and assessing their risks”. It deplored “the premature use of these products in Italy, France and Belgium” and wished “that these imprudences are not repeated on the territory of our Canton”44Annex 2 of the previous document.
Large agglomerates should not necessarily be considered less toxic than small agglomerates
Caution: large agglomerates should not necessarily be considered less toxic than small agglomerates. In early 2020, the results of research conducted in Belgium were published showing that large agglomerates of TiO₂ nanoparticles do not appear to be less active than small agglomerates45Cf. Agglomeration of titanium dioxide nanoparticles increases toxicological responses in vitro and in vivo, Murugadoss S et al, Particle and Fibre Toxicology, 17(10), 2020.
Explore the bibliography below to learn more:
In French :
- Titanium dioxide, Wikipedia
- Opinion on the risk assessment of the nanometric fraction of the food additive E171, Anses, December 2022
- Fiche toxicologique sur le dioxyde de titane, INRS, 2022
- Study of the relationship between titanium dioxide (TiO₂) exposure and cause-specific mortality in a cohort of workers in France A. Gaillen-Guédy, D. Luce, P. Wild, I. Guseva Canu, Archives des Maladies Professionnelles et de l’Environnement, Volume 81, Issue 1, Page 64, February 2020
- Weight of epidemiological evidence in the classification of titanium dioxide by the European Chemicals Agency, I. Guseva Canu, S. Fraize-Frontier, C. Michel, S. Charles, Archives des Maladies Professionnelles et de l’Environnement, Volume 81, Page 71, Issue 1, February 2020
- Toxicological reference values – Titanium dioxide in nanoparticulate form, Anses, January 2019 (made public in April 2019)
- High Council for Public Health (HCSP),
Assessment of knowledge on the effects of titanium dioxide (TiO₂) nanoparticles on human health; characterization of population exposure and management measures
, April 2018 (made public in June 2018) - Impacts of acute oral, or chronic respiratory exposure to titanium dioxide nanoparticles and potential pathophysiological effects in rodents, Jérôme Gay-Queheillard, Bulletin de veille scientifique en sécurité sanitaire de l’environnement et du travail, pp.15-18, Perinatal and Toxic Risks Laboratory, 2018
- Jean-Jacques Perrier, “Le titane : promesses et risques d’un dépolluant”, in La civilisation des nanoproduits, éditions Belin, September 2017
- INERIS, Proposal of a toxicological benchmark for nanoscale titanium oxide for environmental exposures by the respiratory or oral route, study report, November 2016
- Francelyne Marano, Faut-il avoir peur des nanos ?, Buchet Chastel, April 2016
- INRS, Nanoscale titanium dioxide: the need for an occupational exposure limit value, Hygiène et sécurité du travail, n°242, NT 36, March 2016
- Mathilde Biola-Clier, Genotoxicity and impact of titanium dioxide nanoparticles on DNA repair in lung alveolar cells. Thesis of Biochemistry, Molecular Biology. Grenoble Alpes University, February 2016.
- NanoResp, Nanomaterials in food. What functions and applications? What are the risks?October 2015
- Dynamics, reactivity and ecotoxicity of metal oxide nanoparticles in soils: impact on the functions and diversity of microbial communities, Marie Simonin, Ecotoxicology, Université Claude Bernard – Lyon I, 2015.
- L’exposition des travailleurs au dioxyde de titane nanoparticulaire : Évaluation de l’exposition au dioxyde de titane nanoparticulaire et métrologie toxicologique Jean-Paul Morin, Les cahiers de la Recherche, Santé, Environnement, Travail, Nanomatériaux et santé, pp.32-34 ANSES, 2015
In English:
- Titanium dioxide Substance Infocard, ECHA
- Advances in genotoxicity of titanium dioxide nanoparticles in vivo and in vitro, Shi J et al, NanoImpact, 25, 100377, January 2022
- A semiquantitative risk ranking of potential human exposure to engineered nanoparticles (ENPs) in Europe, Li, Y and Cummins, E, Science of the Total Environment, 778, July 2021
- Titanium Dioxide Nanoparticles in Food and Personal Care Products-What Do We Know about Their Safety, Joanna Musial et al, Nanomaterials, June 2020
- Task Force: the “(nano) TiO₂ safety communication”, Damjana Drobne (University of Ljubljana, Slovenia) for the Nanosafety Cluster, February 2020
- Weight of epidemiological evidence for titanium dioxide risk assessment: current state and further needs, Guseva Canu I et al, Journal of Exposure Science & Environmental Epidemiology, 2019
- Penetration, distribution and brain toxicity of titanium nanoparticles in rodents’ body: a review, Zeman T et al, IET Nanobiotechnology, 12(6), September 2018
- Morphological alterations induced by the exposure to TiO₂ nanoparticles in primary cortical neuron cultures and in the brain of rats, Valentini X et al, Toxicology Reports, 2018
- Poorly soluble, low toxicity particles facing review – Classification of titanium dioxide as a carcinogen could have major consequences for related substances too, Chemical Watch, March 2018
- The crystal structure of titanium dioxide nanoparticles influences immune activity in vitro and in vivo, Vandebriel RJ et al, Particle and Fibre Toxicology, 15:9, 2018: between the anatase and rutile forms ofTiO₂ nanoparticles, the rutile ones are more harmful to the immune system when inhaled
- Toxicity assessment of anatase and rutile titanium dioxide nanoparticles: The role of degradation in different pH conditions and light exposure, De Matteis V et al, Toxicology in Vitro, 37: 201-210, December 2016
Any questions or comments? This information sheet compiled by AVICENN is intended to be completed and updated. Please feel free to contribute.
Other news on the topic
Upcoming Nano Agenda

- Scientific conference
- 23rd International conference on Advanced Nanomaterials
- From July 23 to July 25, 2025
- Website: www.advanced-nanomaterials-conference.com

- E-learning program: awareness-raising for personnel who come into contact with nanomaterials during research, formulation, production, maintenance, cleaning, upkeep, etc., as well as safety coordinators or engineers, facility managers, heads of laboratories where nanoparticles are handled.
- Organizers: INSTN Grenoble (CEA)
- On the program:
- 1 – Introduction, definition and characteristics of nanomaterials
- 2 – Toxicity of nanomaterials: the state of knowledge
- 3 – Metrology and characterization of nanomaterials
- 4 – Prevention and protection against nanomaterials in the workplace
- 5 – Quiz: assessment of learning outcomes
- The 2-hour course can be viewed for one month from the date of registration.
- Website: https://instn.cea.fr/…risques-lies-aux-nanomateriaux…

- 10th International Conference on Nanoscience and Technology
- Organized by: Chinese Academy of Sciences and Chinese National Center for Nanoscience and Technology
- Website: http://www.chinanano.org.cn/en/
This sheet was originally created in February 2014
Notes and references
- 1Carcinogenic Hazards from Inhaled Carbon Black, Titanium Dioxide, and Talc not Containing Asbestos or Asbestiform Fibers: Recent Evaluations by an IARC Monographs Working Group, Baan RA, Inhalation Toxicology, 2007, 19(1): 213-228. It should be noted that this classification has been challenged on the grounds that the studies cited were conducted on rats, whose respiratory system is different from that of humans
- 2As early as September 2014, the “Board of Appeal” of the European Chemicals Agency (ECHA) had been seized by nine manufacturers of titanium dioxide (Tioxide Europe / Huntsman, Cinkarna, Cristal Pigment, Du Pont, Evonik, Kronos, Precheza, Sachtleben Chemie GmbH and Tronox Pigments) who refused to provide data requested by ECHA as part of the risk assessment associated with titanium dioxide (including its nanoforms) provided for under REACH. In March 2017, they had won their case on the grounds that the term “nanoform” was not defined in Reach. Cf. Decision of the board of appeal of the European Chemicals Agency, March 2017.
- 3Cf. COMMISSION REGULATION (EU) 2020/217 of 4 October 2019 amending, for the purposes of its adaptation to technical and scientific progress, Regulation (EC) No 1272/2008 of the European Parliament and of the Council on classification, labelling and packaging of substances and mixtures and correcting that Regulation published in the Official Journal of the European Union on 18 February 2020 and Corrigendum to Commission Delegated Regulation (EU) 2020/217 of 4 October 2019 amending, for the purposes of its adaptation to technical and scientific progress, Regulation (EC) No 1272/2008 of the European Parliament and of the Council on classification, labelling and packaging of substances and mixtures and correcting that Regulation OJEU, February 25, 2020
- 4Cf. Guide on the classification and labelling of titanium dioxide, ECHA, September 2021
- 5See in particular:
– Legal action against the classification of titanium dioxide, TDMA, June 4, 2020
– Action brought on 13 May 2020 – Brillux and Daw v Commission (Case T-288/20), General Court of the EU - 6
- 7Cf. TiO2 classification stays, VeilleNanos, 22 February 2023
- 8Cf. How industry can launder a health-risking substance, Social Europe, 8 novembre 2023
- 9Cf. Detection of titanium particles in human liver and spleen and possible health implications, Heringa MB et al, Particle and Fibre Toxicology, 15:15, 2018
- 10Cf. Effects of physicochemical properties of TiO₂ nanomaterials for pulmonary inflammation, acute phase response and alveolar proteinosis in intratracheally exposed mice, Danielsen PH et al., Toxicology and Applied Pharmacology, 386(1), January 2020; these findings qualify those of a 2018 study whose results leaned the other way toward a greater harm of anatases to the immune system when inhaled: cf. The crystal structure of titanium dioxide nanoparticles influences immune activity in vitro and in vivo, Vandebriel RJ et al, Particle and Fibre Toxicology, 15:9, 2018.
- 11Cf. A semiquantitative risk ranking of potential human exposure to engineered nanoparticles (ENPs) in Europe, Li, Y and Cummins, E, Science of the Total Environment, 778, July 2021
- 12Systematic review of mechanistic evidence for TiO2 nanoparticle-induced lung carcinogenicity, Wolf S et al., Nanotoxicology, August 2024
- 13See for example:
– ADEME, Indoor air purification by photocatalysis, Technical notice, September 2020
– Gandolfo A et al, Unexpectedly High Levels of Organic Compounds Released by Indoor Photocatalytic Paints, About. Sci. Technol., 52, 19, 11328-11337, 2018
– Indoor Air Quality Observatory, Photocatalytic scrubbing – Opportunity or threat to indoor air quality, IAQO Bulletin No. 4, June 2012.
– Vivagora, CoExNano, Nanosilver and titanium dioxides in coatings: State of knowledge, uncertainties and controversies, November 2010 - 14Cf. Prasath, S et al., Profiling Nano-Titanium Dioxide Use in Singapore: a Survey of Practices, Quantities, and Workers, Academia Materials Science, 2(1), 2025 or Exposure to nanoscale titanium dioxide in construction and public works, Bertrand Honnert, Archives des Maladies Professionnelles et de l’Environnement, INRS, May 2018
- 15Bonetta et al., DNA damage in workers exposed to pigment grade titanium dioxide (TiO2) and association with biomarkers of oxidative stress and inflammation, Environnemental Toxicology and Pharmacology, Volume 105, November 2023
- 16Cf. Protecting workers and people in the vicinity of titanium dioxide nanoparticle production or handling sites, High Council for Public Health, June 25, 2018
- 17See for example:
In French :
– Doc’ en clip – the risk associated with nanoparticles in sunscreens (video), Riccardo Catalano, Aix-Marseille University, October 14, 2019
– Dynamics, reactivity and ecotoxicity of metal oxide nanoparticles in soils: impact on the functions and diversity of microbial communities, thesis by Marie Simonin (Microbial Ecology / UMR CNRS 5557 Claude Bernard University – Lyon 1), defended in October 2015
– Nano or not: the TiO₂ is toxic for the environment, The Cosmetics Observatory, October (summary in French of the report“Environmental hazard of selected TiO₂ nanomaterials under consideration of relevant exposure scenarios“, Umwelt bundesamt, October 2014).
In English:
– Proteomics reveals multiple effects of titanium dioxide and silver nanoparticles in the metabolism of turbot, Scophthalmus maximus, Araújo MJ et al, Chemosphere, 2022
– Zinc oxide, titanium dioxide and C60 fullerene nanoparticles, alone and in mixture, differently affect biomarker responses and proteome in the clam Ruditapes philippinarum, Marisa I et al, Science of the Total Environment, 838 (2), September 2022
– Toxicity of titanium nano-oxide nanoparticles (TiO2) on the pacific oyster, Crassostrea gigas: immunity and antioxidant defence, Arash Javanshir Khoei and Kiadokht Rezaei, Toxin Reviews, 41, 2022
– Lethal and sub-lethal effects of nanosized titanium dioxide particles on Hydropsyche exocellata Dufour, 1841, Torres-Garcia D et al, Aquatic Insects – International Journal of Freshwater Entomology, 41(1), 2020
– Silver and titanium nanomaterials present in wastewater have toxic effects on crustaceans and fish cells, Norwegian Institute for Water Research (NIVA), November 2019
– Evaluation of the effects of titanium dioxide and aluminum oxide nanoparticles through tarsal contact exposure in the model insect Oncopeltus fasciatus, López-Muñoz D. et al, Science of The Total Environment, 666: 759-765, May 2019
– Titanium dioxide nanoparticles impaired both photochemical and non-photochemical phases of photosynthesis in wheat, Dias MC, Protoplasma, 256(1): 69-78, January 2019
– Transfer and Ecotoxicity of Titanium Dioxide Nanoparticles in the Terrestrial and Aquatic Ecosystems: A Microcosm Study, Vijayaraj V et al, Environmental Science and Technology, 52(21): 12757-12764, October 2018
– Toxicological impact of TiO₂ nanoparticles on Eudrilus euginiae, Priyanka KP et al, IET Nanobiotechnology, 12 (5):579, August 2018
– Ecotoxicological Effects of Transformed Silver and Titanium Dioxide Nanoparticles in the Effluent from a Lab-Scale Wastewater Treatment System, Georgantzopoulou A et al., About. Sci. Technol., 52, 16, 9431-9441, 2018
– Environmental exposure to TiO₂ nanomaterials incorporated in building material, Bossa N et al, Environmental Pollution, January 2017
– Titanium dioxide nanoparticles strongly impact soil microbial function by affecting archaeal nitrifiers, Simonin M et al, Scientific Reports, 6, 2016
– Ecotoxicity of engineered TiO₂ nanoparticles to saltwater organisms: An overview, D. Minetto, G. Libralato, A. Volpi Ghirardini, Environment International, May 2014 - 18See the paragraph “From 2007 to 2017, ten years of arm wrestling over nanomaterials regulation (or lack thereof) under REACH” on our information sheet How are nanomaterials regulated under REACH? and the page Anses’ actions on titanium dioxyde (in French) on Anses website, 18 october 2024
- 19Cf. Titanium dioxide: E171 no longer considered safe when used as a food additive, EFSA, 6 mai 2021
- 20Cf: WHO Technical Report Series (TSR 1051)
- 21See in particular Les nanoparticules de dioxyde de titane, leur place dans l’industrie cosmétique et ses dangers, Laura Daragnes, Thèse pour l’obtention du diplôme d’Etat de docteur en pharmacie, sous la direction de Isabelle Bestel, Université de Bordeaux, September 2018
- 22(82 our of 84)
- 23(RM09 and RM11, for which the provided genotoxicity data indicate no genotoxicity concern)
- 24CSAS issued an initial opinion on July 23, 2013, revised in 2014: Scientific Committee on Consumer Safety SCCS OPINION ON Titanium Dioxide (nano form) COLIPA No. S75, SCCS, April 2014 and completed September 2014: Scientific Opinion for clarification of the meaning of the term “sprayable applications/products” for the nano forms of Carbon Black CI 77266, Titanium Oxide and Zinc Oxide, SCCS, September 2014 (published June 2015)).
- 25Cf. Our sheet Framing of nanomaterials in cosmetics, veillenanos.fr .
- 26In 2012, researchers in Cincinnati, USA, showed that chlorine from swimming pools can degrade the aluminum hydroxide coating that surrounds titanium dioxide (TiO₂) nanoparticles embedded in some sunscreens (here Neutrogena SPF 30). In contact with water and under the effect of light, the core of the nanomaterial, the nano TiO₂ can then release free radicals, which are responsible for skin aging and the development of cancers
– Cf. Depletion of the protective aluminum hydroxide coating in TiO2-based sunscreens by swimming pool water ingredients, Chemical Engineering Journal, 191: 95-103, May 2012
– See also this more recent article: UV filters interaction in the chlorinated swimming pool, a new challenge for urbanization, a need for community scale investigations, Sharifan H et al, Environ Res, 148:273-276, July 2016 - 27In 2014, Spanish researchers estimated that tourist activity on a Mediterranean beach during a summer day can release about 4 kg of titanium dioxide nanoparticles into the water, resulting in an increase of 270 nM/day in the concentration of hydrogen peroxide (a molecule with toxic potential, particularly for phytoplankton, which is the basic food of marine animals). Cf. Nanos UV screens: a danger for marine life, L’Observatoire des Cosmétiques, September 5, 2014
– In 2017, researchers from CEREGE in France, on the other hand, measured the concentration of titanium in the water of three beaches in Marseille and estimated the weight of TiO₂ released in the two summer months for one small beach at 54 kilograms per day. See :
– Scientists find titanium dioxide from sunscreen is polluting beaches Scientists find titanium dioxide from sunscreen is polluting beaches, presentation by Labille J., Goldschmidt Conference, August 2018
– Estimation and minimization of the risk associated with TiO2 nanoparticles used in sunscreens, presentation by Labille J, “Nano and cosmetics” technical day organized by LNE, 29 March 2018
– Pollution of coastal waters by UV absorbers from sunscreens, generated by summer activities, Labille J, OHM Littoral project, 2017 - 28See in particular:
– Identification of titanium dioxide on the hair shaft of patients with and without frontal fibrosing alopecia: a pilot study of 20 patients, CT Thompson, ZQ Chen , A Kolivras, A Tosti, British Journal of Dermatology, 2019
– Titanium dioxide nanoparticles and frontal fibrosing alopecia: cause or consequence?, O. Aerts, A. Bracke, A. Goossens, V. Meuleman, J. Lambert, Journal of The European Academy of Dermatology and Venereology, June 2018
– Sunscreen, nanoparticles and frontal alopecia, Synchrotron sun, February 2018
– Detection of titanium nanoparticles in the hair shafts of a patient with frontal fibrosing alopecia, Brunet-Possenti F, Deschamps L, Colboc H, et al, Eur Acad Dermatol Venereol, 2018. - 29Cf. NanoTiO2 Sunscreen Does Not Prevent Systemic Oxidative Stress Caused by UV Radiation and a Minor Amount of NanoTiO2 is Absorbed in Humans, Pelclova D et al, Nanomaterials, 9(6), 888, 2019
- 30Titanium dioxide in organic cosmetics, Cosmebio, March 2019
- 31See in particular:
– Sunscreen: FDA regulation, and environmental and health impact, Shanthi Narla, Henry W. Lim, Photochemical & Photobiological Sciences, 2020
– Titanium Dioxide and Zinc Oxide Nanoparticles in Sunscreens: A Review of Toxicological Data, Vujovic M, Kostic E, Journal of Cosmetic Science, September 2019
– Fueling a Hot Debate on the Application of TiO2 Nanoparticles in Sunscreen, Sharma S et al, Materials, July 2019 - 32See for example:
– Sunscreen products for children – Too many substances of concern, Agir pour l’Environnement and WECF France, July 2020
– Titanium dioxide nanoparticles, their place in the cosmetic industry and its dangers, Laura Daragnes, Thesis for the obtention of the State Diploma of Doctor of Pharmacy, under the direction of Isabelle Bestel, University of Bordeaux, September 2018
– Nanoparticles – Attention, elles se cachent partout, Que Choisir, Mensuel n° 566, February 2018 - 33See our fact sheet“Towards the suspension of titanium dioxide in cosmetics and medicines” for more information.
- 34See for example:
– Evaluation of the Performance and Durability of Self-Cleaning Treatments Based on TiO2 Nanoparticles Applied to Cement-Based Renders and Boards, Fregni, A, Venturi, L, Franzoni, E, Coatings, 13, 990, 2023
– Photocatalytic TiO2-Based Coatings for Mortars on Facades: A Review of Efficiency, Durability, and Sustainability, Bersch, JD et al, Buildings, 13, 186, 2023
– Paints to purify ambient air, CEA Liten, November 2020 - 35The Centre Scientifique et Technique du Bâtiment (CSTB) and the LNE (MONA Platform) participated in the aeraulic characterization phase of this bench and in the physicochemical characterization of the nano-objects emitted in the exposure chamber. Then ANSES and the CarMeN laboratory were involved for the inhalation exposure and in vivo analysis
- 36See in particular: In vivo evaluation of the potential neurotoxicity of aerosols released from mechanical stress of nano-TiO2 additived paints in mice chronically exposed by inhalation, Maxinay S et al., J. Phys: Conf. Ser, 838 012025, 2017
Research Activity Report 2016, LNE, 2016 - 37Research into the safety of nanoparticles – No nano-dust danger from façade paintEMPA, January 13, 2014; subsidized to the tune of 2.4 million euros by the European Commission, out of an overall budget of 3.1 million euros, the NanoHouse project ran from January 2010 to June 2013, with French partners the CEA et ISTerre.
- 38Cf. Emission of titanium dioxide nanoparticles from building materials to the environment by wear and weather, Shandilya, N et al, Environmental Science & Technology, 49(4): 2163-2170, 2015; a lay summary is freely available here : Nanocoating on buildings releases potentially toxic particles to the air, “Science for Environment Policy,” European Commission, May 28, 2015
- 39Cf. Interim report – elements for environmental metrological monitoring of titanium dioxide (TiO₂) nanoparticles and feasibility review, HCSP, October 2019 (publication June 2020)
- 40Cf. Arrêté portant prescriptions complémentaires à la société CRISTAL France SAS à Thann, Préfecture du Haut-Rhin, 3 August 2018 ; see also Estimation of average annual concentrations in the air around an industrial site producing substances in the nanoparticulate state – Cristal site – Thann, Titanium dioxide production unit, INERIS, October 2017
- 41
- 42Cf. Agglomeration of titanium dioxide nanoparticles increases toxicological responses in vitro and in vivo, Murugadoss S et al, Particle and Fibre Toxicology, 17(10), 2020
- 43Health: straight into the wall… self-cleaning, Alternative Santé, January 6, 2016 and Report M 1741-A of the State Council to the Grand Council of Geneva, 2008.
- 44Annex 2 of the previous document
- 45Cf. Agglomeration of titanium dioxide nanoparticles increases toxicological responses in vitro and in vivo, Murugadoss S et al, Particle and Fibre Toxicology, 17(10), 2020.







