
Our product tests

Our product tests
By the AVICENN team – Last modification January 2026
Context and objectives
Our tests are part of an effort to make a concrete contribution to the 4th National Health and Environment Plan (PNSE 4) published in 2021, whose action #13 aims in particular to:
- improve knowledge about the use of nanomaterials
- monitor and extend labeling requirements for nanomaterials in everyday consumer products
- improve knowledge about the health and environmental risks associated with nanomaterials
- regulate nanomaterials that do not provide significant benefits and may pose risks.
In 2020-2021 — our tests of “air purifier” IKEA curtains
In February 2019, the IKEA brand launched a promotional campaign for future “air-purifying” curtains.
Suspecting the presence of titanium dioxide nanoparticles, AVICENN contacted the famous Swedish brand and, in the absence of any response, had the products tested:
- analyses carried out by the LNE showed a massive presence of titanium dioxide nanoparticles on the surface of the fibers;
- analyses conducted by IRCELYON showed that they had no depolluting (air-purifying) effectiveness.
→ In the first half of 2021, IKEA withdrew from the market its new GUNRID “air purifier curtains”.
In 2022 — Our tests on 23 everyday products
In September 2021, we decided to launch tests on about twenty everyday consumer products, with the primary objective of ascertaining the presence (or not) of nanoparticles of concern in everyday products.
Our approach
Our investigation took place over 15 months:
- Between September and October 2021, we defined the criteria for product selection: products likely to contain nanoparticles with well-documented hazards, cause frequent/chronic exposure, be sold on a large scale, affect various populations, etc.
- We then targeted specific products:
- by soliciting our members and subscribers,
- by carrying out documentary reviews (veillenanos.fr, the NanoDatabase, online sales sites > labeling, technical data sheets, SDS)
- By scouting in supermarkets
- By asking public authorities, research laboratories and brands
- At the end of 2021 / beginning of 2022, we sent about 20 products to the National Laboratory of Metrology and Testing (LNE).
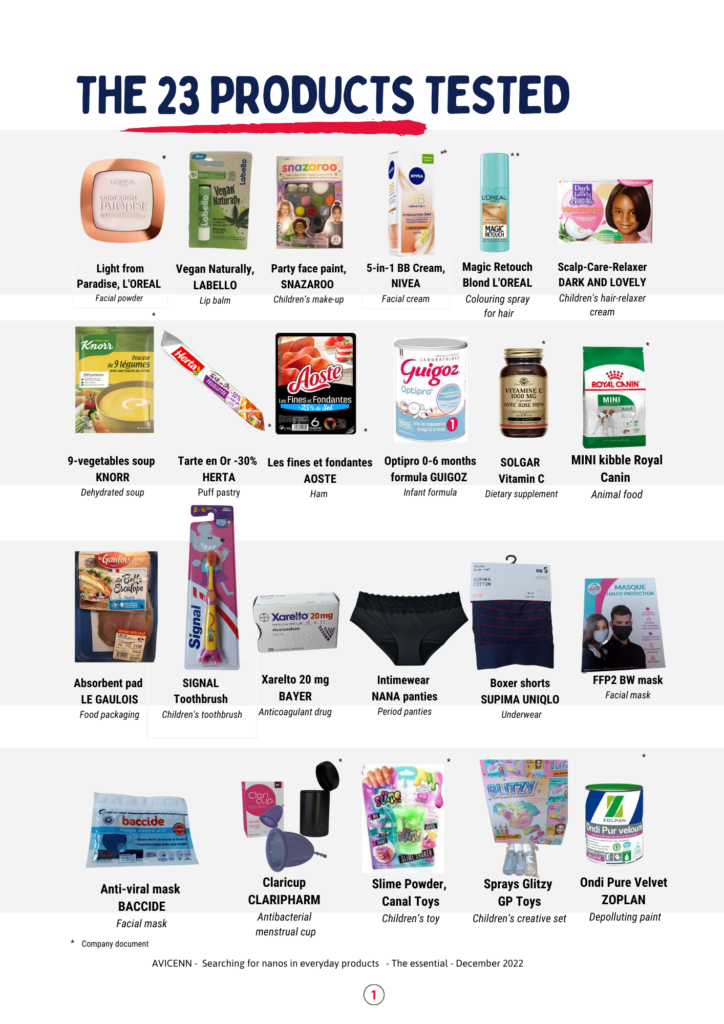
- The tests were carried out between the end of 2021 and the end of August 2022 at LNE, with the final analysis report received mid-October 2022.
- AVICENN published its report “In search of [nanos] in everyday products” on December 15, 2022. The tests reveal the presence of unlabeled, sometimes even unauthorized, nanos in 20 of the 23 everyday products tested:

The results: nanos found in 20 products out of 23
Nanos have been detected in 20 products.
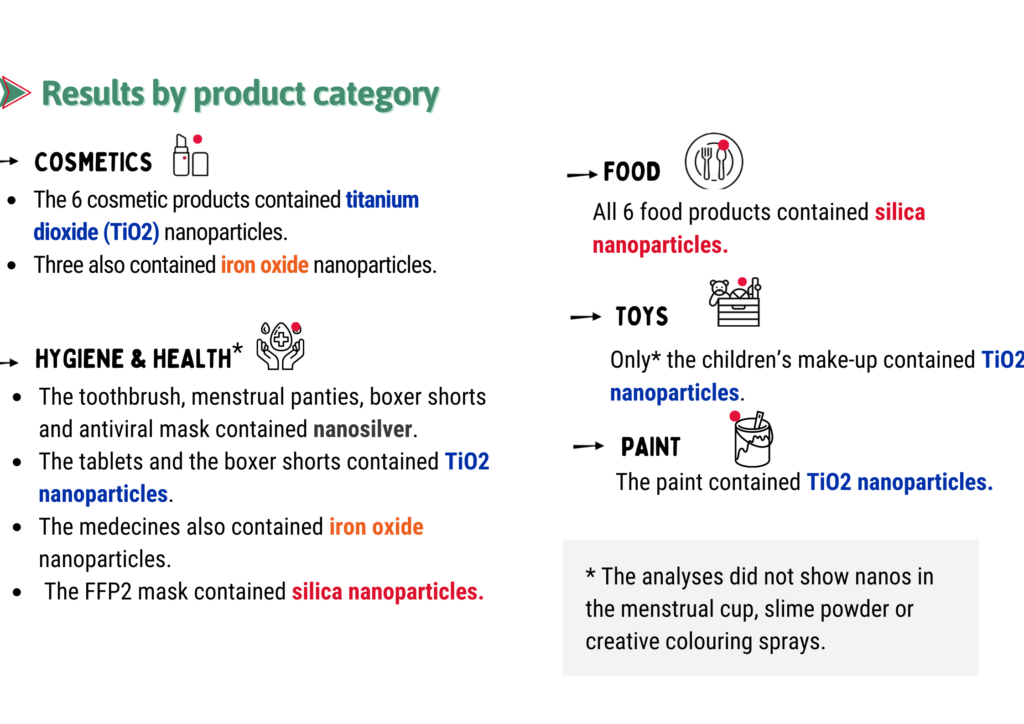
Results by product category:
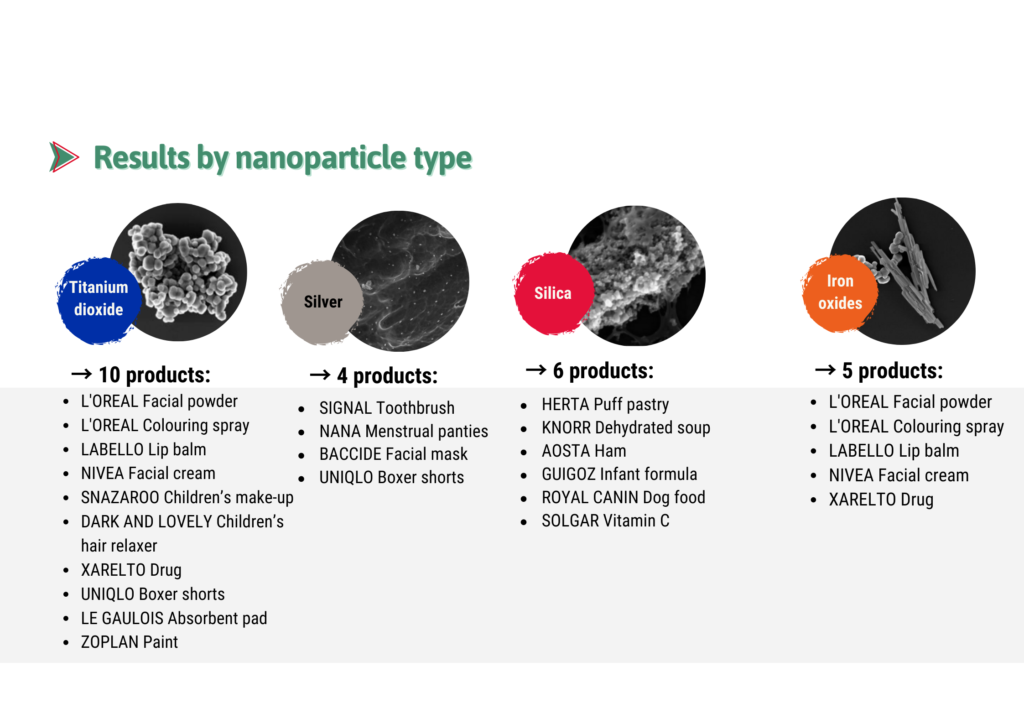
Results by type of nanoparticles:
More nanos than expected and some new questions…
We selected products that, although not labeled “nano”, were more likely than others to contain nanos. And yet we were surprised by the high proportion of products containing nanos as well as discovering some where we did not expect them.
These results raise questions about the extent of the use of nanos in everyday products and show the relevance – and even the necessity – of using the new tools available and innovative approached to look for nanos in commonly used items.
Assessment of the [nano] labeling: a complete failure!
The results show how eminently flawed the [nano] labeling is.
None of the products in which we have detected nanos were labeled [nano] even though most of them are covered by the European obligation of “nano” labeling, which has been in force for almost ten years for cosmetics, food products and biocides.
The presence of nanos in other product categories also underlines the need to extend the [nano] labeling obligation to product categories that are unfortunately still not currently concerned.
Many unauthorized nanos
Our tests have also highlighted, to an unsuspected extent, the fact that nanos are commonly used even though they are not authorized. This is particularly the case in cosmetics and hygiene and health products.
They talk about our investigation
In the media
- European and international press: Chemical Watch, RFI, BBN World News, Food Navigator, California 18, Il Salvagente, Il Ssusidario, Psychomédia, RTBF, the NanoDataBase, …
- French national media : France inter (7 a.m. news on December 15), France info web, radio and TV, Le Monde, What to choose, 60 millions de consommateurs, Liberation, The Tribune, 20 Minutes, Le Figaro, Les Echos, La Croix, The Express, BFM, Ecoréseau, Syndicalisme Hebdo, Novethic, Reporterre, GEO, Planet, HelloDoctors, Santé Magazine, I’m interested, Medisite, Why Doctor, Femina, Femme actuelle, Madame Figaro, Marie-Claire, …
- French regional media : France Bleu, Ouest France, Nice Matin, Var Matin, La Dépêche, …
At the National Assembly
Following our tests, four written questions were put to the government:
- On December 27, 2022, in his question n°4492 written to the attention of the Minister of Health, the Renaissance deputy Guillaume Vuilletet invoked “the need to systematize the research and for a ban of nanoparticles in everyday products”. The MP believes that “the medium- and long-term health risks seem to be largely underestimated, especially in the case of chronic exposure to products containing several nanoparticles in large quantities, such as certain cosmetic products. Mr. Vuilletet further emphasized that “The precautionary principle is essential: consumer exposure to nanomaterials must be clearly limited until their safety has been demonstrated. He asked the Minister about his willingness to“intensify controls and sanctions for companies that do not comply with the labeling obligation and the relevance of implementing the recommendation of AVICENN, which proposes that manufacturers participate in the financing of independent research to better assess the risks associated with nanomaterials”.
- Three other questions were subsequently asked by other members:
- On January 31, 2023, in his written question n°5046, Renaissance Deputy Karl Olive has called for an assessment of “the current regulations and possible evolutions”.as well as “the means implemented by the Ministry and the Directorate of Consumer Affairs and Fraud Control to better control the use of these particles and in particular titanium dioxide” and the “means of informing the consumer about these nanoparticles via labelling in particular”.
- On February 14, 2023, in her written question No. 5449, the deputy Horizons Agnes Carel also asked to know “what provisions are planned to better control the use of these particles and in particular titanium dioxide and better inform the consumer of their presence in the products they consume.
- On February 21, 2023, in his written question n°5701, Renaissance MP Vincent Ledoux asked the government to “please tell him what he intends to implement in order to better control the presence of these nanoparticles in everyday products, which constitute a worrying public health problem”.
Since the publication of our tests
Over the months, several of the tested products or identified nanoparticles have been removed from store shelves:
- IKEA has withdrawn its GUNRID curtains, which contained titanium dioxide nanoparticles.
- Aoste now offers nitrate-free ham, which is also free of silica nanoparticles.
- Nana has launched a new cotton underwear, which the company assured us is free of nanosilver (and silver).
- Signal has removed silver nanoparticles from its toothbrushes (and titanium dioxide from its toothpastes).
- Guigoz certifies that its powdered milk and other products no longer contain silica nanoparticles.
- The “Light from Paradise” powder by L’Oréal is no longer available for purchase on L’Oréal’s website and has disappeared from store displays and websites of several retailers where it was previously sold.
The report is available in both English and French
The English version of our report was released at the conference organized by the European NGO ECOS on February 15, 2023, entitled ” Unnoticed and ungoverned: How nanomaterials are slipping through the cracks ” (and relayed by One Policy Place – OPP).
In 2024-2025 — Our tests on 10 pearlescent cosmetics
At the end of 2025, AVICENN published a new investigative report “Searching for [nanos] in glitter cosmetics”: the 10 glitter cosmetics we had tested contain very large quantities of unauthorized and particularly small nanoparticles of titanium dioxide (TiO2), potentially dangerous to health.
Thanks to highly advanced analyses and images of unprecedented precision, our tests clearly show TiO2 nanoparticles detached from the mica sheets, which are visibly cracked, crumbling or even broken, releasing nanoparticles that can be inhaled and cause adverse effects in the event of repeated exposure.
The French National Health and Safety Agency (ANSES) has begun working on this issue, following a decision by the Ministry of Health, to whom we communicated our results.
→ More information to come and here.
Summary of AVICENN’s requests
Because of the health and environmental risks associated with nanomaterials, AVICENN makes three requests at the end of the report:
1 – to improve knowledge and awareness of marketed nanomaterials, in particular by improving the French register (ten years after its creation, this register remains a real sieve) and the implementation of a European register of products containing nanos and to better evaluate their risks, by making the companies that import, produce or use them contribute to the analyses;
2 – increase transparency on these nanomaterials with, among other things, the intensification of controls and sanctions in case of non-compliance with legal obligations (whether it be labelling, registration, and/or authorization);
3 – and finally, increase vigilance with the introduction of a generalized obligation for an assessment of the benefit/risk ratio and collective utility of nanomaterials BEFORE they are put on the market and with the implementation of specific measures concerning nanos that escape the new European recommendation for the definition of the term “nanomaterial“, so that many nanos do NOT go off the radar of public authorities.
Any questions or comments? This information sheet compiled by AVICENN is intended to be completed and updated. Please feel free to contribute.
Our information sheets to go further
Upcoming Nano Agenda

- International conference on metallic nano-objects for experts working in the interdisciplinary field of metallic nanoparticles, with a particular emphasis on nanoparticle synthesis and characterization, plasmonics, optics and photonics, catalysis, biomedicine, electronics, and nanoparticle recycling
- Organizers / Partners: CNRS, Bordeaux University, Bordeaux INP, ICMCB, CRPP, CBMN, ISM
- Website: https://mno2026.sciencesconf.org

- Seminar presenting the Seirich system for chemical risk assessment, with a focus on the specificities of nanomaterials.
- Organizers: Association NanoMesureFrance
- Speakers: Florian Marc (INRS)
- Website: www.eventbrite.fr/e/billets-nanomesurefrance-webinar-1984136502691
- Toxicokinetics: the fate of chemicals in the body” training course:
- the different routes of entry for toxic products
- the importance of toxicokinetics in preventing substance toxicity,
- xenobiotic absorption, distribution, metabolism and elimination
- nanoparticle toxicity
- Organizer: Association Toxicologie Chimie (ATC)
- Date: March 26, 2026
- Speaker: Nicole Proust (Research Engineer, CNRS Honorary Research Director, Palaiseau)
- Website: www.atctoxicologie.fr/…
File originally created in January 2022



