
Definition of nanomaterials in food: MEPs say no to Commission draft
During their very last plenary session in Strasbourg, MEPs rejected the European Commission’s plan to incorporate its recommended definition of “nanomaterials” into “novel foods” regulation, criticized in particular because of the threshold of 50%, too high. It is now invalidated.
Barely adopted, the Commission’s new definition has just been buried
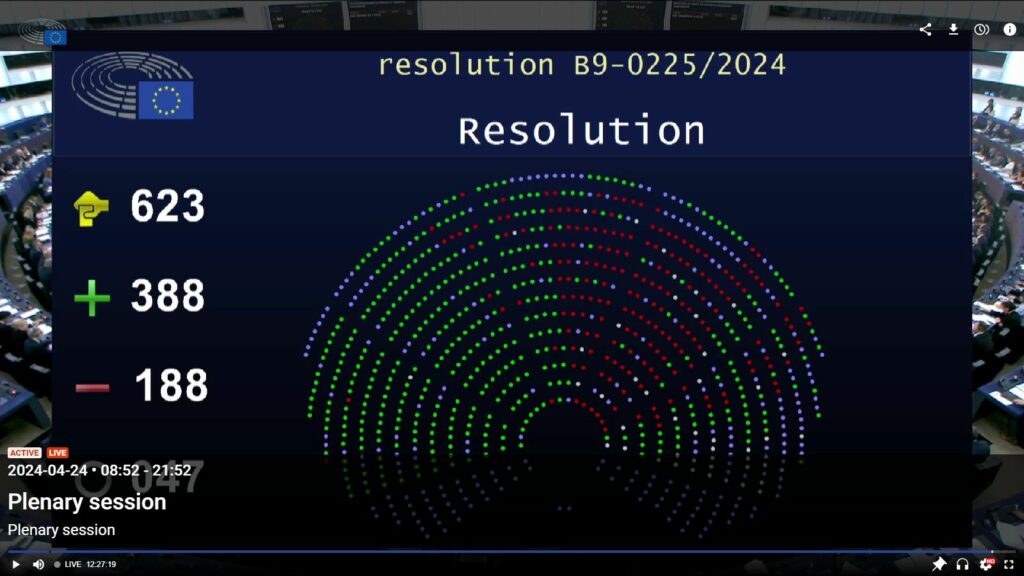
On Wednesday April 24, the plenary session of the European Parliament in Strasbourg rejected by over 62% the definition of “nanomaterial” that the European Commission wanted to include in European food legislation. More specifically, MEPs voted in favor of a resolution rejecting the delegated act adopted by the European Commission on March 14, which aimed to incorporate the Commission’s 2022 recommendation for a definition of the term “nanomaterial” into the Novel Foods Regulation, in order to replace the current definition1Contained in Regulation (EU) 2015/2283 of the European Parliament and of the Council on novel foods of November 25, 2015, the current definition of a nanomaterial is as follows: “intentionally produced material that has one or more dimensions of the order of 100 nm or less or that is composed of discrete functional parts, either internally or at the surface, many of which have one or more dimensions of the order of 100 nm or less, including structures, agglomerates or aggregates, which may have a size above the order of 100 nm but retain properties that are characteristic of the nanoscale”.. The Parliament has thus asserted its right (shared with the Council) to lodge an objection to the Commission’s delegated acts, within two months of their publication.
Ten years ago, the European Parliament rejected a similar project.
Back in March 2014, the MEPs had already voted a resolution rejecting a delegated act with a very similar definition (including a minimum threshold of 50% in number of particles smaller than 100 nm) because they had judged that the latter “run contrary to (…) a high level of protection of consumers’ health and interests”.
In the same text, the Parliament also asked the Commission to submit a new delegated act taking account of its position. And yet, the new proposal submitted by the Commission just over a month ago in response to this request closely resembled, with a few exceptions, the definition rejected by the Parliament ten years ago, notably with the same 50% threshold.
The proposed definition and its 50% threshold had been criticized – unsuccessfully until now
Wednesday’s vote therefore puts a serious stop to the European Commission’s project, after a long process during which the Commission turned a deaf ear to the arguments put forward by health authorities and associations. It had not taken into account the arguments put forward by numerous organizations during the public consultations in 2021 and 2023/2024.
The aim pursued by the European Commission since its first definition recommendation in 2011 has always been to impose a definition with a 50% threshold, not only in the REACH regulation and the biocides regulation – where this figure was introduced – but also in the novel foods and cosmetics regulations, in which MEPs did not include a minimum threshold for particles below 100 nm.
French authorities, in line with the goal of MEPs to protect consumer health and information, have applied a 10% “tolerance” when controlling [nano] labelling in food and cosmetics. This rate, which is more protective than the 50% rate, ensures that substances containing, for example, ~49% nanoparticles are not considered nor labeled as “nano”. The European Commission, for its part, has never seen fit to justify why the 50% threshold was, in its view, more appropriate2(other than by asserting that below 50%, nanoparticles are not in the majority and that, consequently, the ingredient of which they are part is not predominantly a “nanomaterial”).
AVICENN had responded to the consultations3Click here to read our contribution posted on January 12, 2024 on the Commission’s website, along with other NGOs, ANSES, the French authorities, etc. to voice their concerns and opposition to the 50% rate in particular, as well as to other changes introduced by the Commission in 2022 that could undermine consumer information and health. Surprisingly, the ANSES’ well-documented report on the subject published last year has been completely ignored by the European Commission, which never made a single reference to the report – either in the document submitted for consultation at the end of 2023 nor in the delegated act it published in mid-March 2024.
MEPs defend consumer information
On April 18, during a first ENVI commission vote, then on April 24 at the last plenary session of the current term, European deputies voted in large numbers – in a proportion of more than two to one – a resolution rejecting the European Commission’s delegated act with the new definition. This cross-party resolution was prepared by Jutta Paulus (Les Verts, ALE), Christel Schaldemose (S&D), Sirpa Pietikäinen (PPE), Frédérique Ries (RENEW) and (Anja Hazekamp, The Left).
This vote was supported by foodwatch, BEUC and AVICENN, who, along with ANSES and the French authorities in particular, share the concern to see the mandatory [nano] label protected, as requested by 87% of Europeans. With the 10% threshold, France has set an example that should be followed. Immediately after the vote, BEUC tweeted that “member states must better enforce current rules on nanofood labeling”.
As for companies tempted “to hide behind an unintentional character of the presence of nanomaterials in food“ (as mentioned by the European Commission’s Bruno Gautrais during his presentation to the ENVI commission on April 18), BEUC warned: they “can count on consumer groups to test products for undeclared nanoparticles“.
→ Useful links :
– Procedure file (on the European Parliament website)
– Information on the rejected delegated act (on the Commission website)
– Page on nanomaterials in food (on the Commission website)
– Our previous articles dealing with this issue of the definition of “nanomaterials” at European level
Les prochains RDV nano

- Scientific conference
- 23rd International conference on Advanced Nanomaterials
- From July 23 to July 25, 2025
- Website: www.advanced-nanomaterials-conference.com

- E-learning program: awareness-raising for personnel who come into contact with nanomaterials during research, formulation, production, maintenance, cleaning, upkeep, etc., as well as safety coordinators or engineers, facility managers, heads of laboratories where nanoparticles are handled.
- Organizers: INSTN Grenoble (CEA)
- On the program:
- 1 – Introduction, definition and characteristics of nanomaterials
- 2 – Toxicity of nanomaterials: the state of knowledge
- 3 – Metrology and characterization of nanomaterials
- 4 – Prevention and protection against nanomaterials in the workplace
- 5 – Quiz: assessment of learning outcomes
- The 2-hour course can be viewed for one month from the date of registration.
- Website: https://instn.cea.fr/…risques-lies-aux-nanomateriaux…

- E-learning program: awareness-raising for personnel who come into contact with nanomaterials during research, formulation, production, maintenance, cleaning, upkeep, etc., as well as safety coordinators or engineers, facility managers, heads of laboratories where nanoparticles are handled.
- Organizers: INSTN Grenoble (CEA)
- On the program:
- 1 – Introduction, definition and characteristics of nanomaterials
- 2 – Toxicity of nanomaterials: the state of knowledge
- 3 – Metrology and characterization of nanomaterials
- 4 – Prevention and protection against nanomaterials in the workplace
- 5 – Quiz: assessment of learning outcomes
- The 2-hour course can be viewed for one month from the date of registration.
- Website: https://instn.cea.fr/…risques-lies-aux-nanomateriaux…
Notes and references
- 1Contained in Regulation (EU) 2015/2283 of the European Parliament and of the Council on novel foods of November 25, 2015, the current definition of a nanomaterial is as follows: “intentionally produced material that has one or more dimensions of the order of 100 nm or less or that is composed of discrete functional parts, either internally or at the surface, many of which have one or more dimensions of the order of 100 nm or less, including structures, agglomerates or aggregates, which may have a size above the order of 100 nm but retain properties that are characteristic of the nanoscale”.
- 2(other than by asserting that below 50%, nanoparticles are not in the majority and that, consequently, the ingredient of which they are part is not predominantly a “nanomaterial”)
- 3Click here to read our contribution posted on January 12, 2024 on the Commission’s website









