Towards the ban on titanium dioxide in cosmetics and medicines?

After the ban in foodstuff, will titanium dioxide be banned in cosmetics (except sunscreens) and medicines too?
By AVICENN – Last addition August 2025
Titanium dioxide (TiO2) is a white colorant in powder form, with a large number of nanoparticles1smaller than 100 nanometers (1 nm = 0.001 µm = 1 billionth of a meter). It has been prohibited in foodstuffs in France in 2020, an in the entir ie European Union in 2022, following suspicions of colon carcinogenicity and of potential genotoxic risks.
- Regarding cosmetics, for now, titanium dioxide is still allowed at the non-nano scale for other oral cosmetic uses, such as toothpastes or lipsticks and balms (where it can be identified by the code CI 77891). An opinion from the European Scientific Committee on Consumer Safety (SCCS), first expected in March 2023, is now expected… after June 2025.
- Regarding medicinal products, the European Commission has laid the groundwork for a potential ban that could come into effect from 2025. Emphasizing that “it is crucial that the pharmaceutical industry makes every effort to accelerate research and development of alternative solutions to replace titanium dioxide (E171) in medicines”2See recitals 14 to 18 and article 3 of the European regulation 2022/63..
The ban of (nano)particles of titanium dioxide in consumer products has been requested for about ten years by various organizations (associations, journalists, health agencies, …). These demands have accelerated recently with the ban of titanium dioxide in foodstuffs (E171) in 2020.
AVICENN is compiling below the requests in favor of the extension of this ban to cosmetics likely to be ingested and/or inhaled (toothpastes, make-up, lip balms…) and to medicines.
Chronology:
In 2025
- 25 August 2025: The petition launched last March on the MesOpinions.com platform calling on the French Ministry of Health and the European Parliament to “ban titanium dioxide in medicines” has exceeded 20,000 signatures.
- 6 August 2025: A Staff Working Document published by the European Commission on August 6 states that “the authorization of E171 as a colorant in medicinal products should be maintained” while also noting later that this opinion is based on “the limited set of data submitted by the industry consortium and not on a comprehensive review by the European medicines agency (EMA) of all available data”3Cf. Authorization of E171 in medicines “should be maintained”, at the request of pharmaceutical companies, AVICENN @VeilleNanos, 14 août 2025…... despite a growing number of publications on the harmful effects of E171.
- 23 July 2025 : Despite the ban on E171 that has been in place since 2020 in France, titanium dioxide nanoparticles have been detected in human, animal, and infant milks by French researchers from INRAE, AP-HP, Synchrotron Soleil, and CNRS.
→ Le Monde newspaper who sought AVICENN’s advice, reports our comments: “These results are very worrying. The precautionary principle should lead the authorities to suspend the authorization of this substance for non-essential uses likely to lead to environmental and human exposure”. Starting with toothpastes and medications. - 26 June 2025 : During the 11th Plenary meeting 2022-2026 of the Scientific Committee on Consumer Safety (SCCS), the mandate on TiO2 (nano and non-nano) was not adopted, as the full safety file, which should have been sent by the industrial consortium, had not been received yet.
- June 2025: Following the publication of our article ‘E171 in pharmaceuticals: when will the European Commission end its silence?‘, other media have covered the issue of E171 in medicines:
- Titanium dioxide in medicines: what is Europe doing?, 60 millions de consommateurs, June 2025
- The European Commission called to account for its inaction, Que Choisir, June 2025
- Titanium dioxyde in drugs: patients no longer want it, Le Moniteur des Pharmaciens, June 2025
- Banned in food, authorised in medicines : is titanium dioxide safe or not?, 20 Minutes – Le Journal des Seniors, June 2025
- 7 daily medicines that contain titanium dioxide, MédiSite, June 2025
- “It’s very vicious”: the presence of titanium dioxide E171 in certain medicines highlighted, France inter, June 2025
- Titanium dioxide: banned in food because it is dangerous, why is it still allowed in medicines?, Sud Ouest, June 2025
- This carcinogenic additive is banned in food, but still authorized in thousands of medicines, Femme actuelle, June 2025
- Banned in food, this carcinogenic additive is still present in all these medicines, Marie France, June 2025
- Titanium Dioxide: For Big Pharma Profits, Lutte ouvrière, July 2025
- Le Monde, juillet 2025.
- …
- April 2025: Regarding cosmetics, during the April 7 meeting of the SCCS’s working group on nanomaterials in cosmetics, the SCCS communicated to the industrial consortium that the clustering methodology outlined in the updated dossier submitted in February was neither sufficiently clear nor robust for TiO2 safety assessment. Additional slides from industry were sent afterwards, before the April 25 meeting where the Consortium presented their refined clustering approach.
The European Commission confirmed that the updated dossier should be submitted by the end of June 2025 at the request of several Member States4More details in the Minutes of the WG on Nanomaterials in Cosmetic Products, SCCS, 7 and 25 April 2025, anxious to achieve a timely decision on the safety of this cosmetic ingredient.
→ As a reminder, SCCS’ opinion was first supposed to be finished in 2023. But with each extension granted to the industry to provide more reliable data, another two and a half years have passed during which cosmetics containing TiO₂ have continued to be marketed—without solid proof of their safety. - February 2025: Regarding pharmaceuticals, according to recitals 14 to 18 and article 3 of European Regulation 2022/63, the European Commission should decide whether to delete – or, if not, to extend for a period to be determined – the authorization for the use of titanium dioxide (E171) in medicinal products.
In 2024
- October 2024 : Senator Alain Marc of the Independents sent a written question to the Minister of Health regarding the carcinogenic nature of E171, which is contained in many medications. He pointed out that E171, “used to bleach medications“, “has no therapeutic effect” and asked her to inform him of her intentions in this matter.
- September 2024: ANSES’ Scientific and Technical Support NOTE on the analysis of the data provided in the context of the assessment of titanium dioxide (TiO2) in cosmetic products is sent to French authorities. This note states that ANSES supports the SCCS’s assessment that the available evidence is not sufficient to exclude the genotoxicity potential of TiO2 used in cosmetic products and that further information is needed in order to consider that cosmetic products containing TiO2 are safe for use.
- 27 June 2024: A survey in Italian supermarkets and pharmacies shows the presence of titanium dioxide in 41 toothpastes.
- 20 May 2024: A scientific article published in Advanced Science demonstrates that, even when filtered by the placenta and not reaching the embryo or fetus, nanoparticles of titanium dioxide (TiO2) and silica (SiO2) pose health risks for the unborn child, in particular poor blood vessel development (see our article here).
→ Among the potential consequences : increased risk of miscarriage, pre-eclampsia, low birth weight, autism, respiratory illnesses, etc.
- 13 May 2024 : In its Scientific Advice on Titanium dioxide (TiO2) 1661/23, the European Scientific Committee on Consumer safety (SCCS) considers that the available evidence, provided by industry for 44 pigmentary et 40 nano TiO2 grades, is not sufficient to exclude the genotoxicity potential of almost all of the types of TiO2 grades5(82 our of 84) used in oral cosmetic products and thus, that more experimental data are needed to exclude the genotoxicity potential of the grades of TiO2 (both pigmentary and nano) used in oral cosmetic products, except for two nano grades6(RM09 and RM11, for which the provided genotoxicity data indicate no genotoxicity concern).
The SCCS also adds that, considering that oral mucosal cells are prone to the uptake of nanoparticles (including TiO2 nanoparticles) and that some oral products containing TiO2 nanoparticles such as toothpastes, will be used every day and potentially more than once a day, further investigations are needed to exclude the risk to the consumer from longterm repeated exposures of the oral mucosa to TiO2 nanoparticles. - 2 May 2024: CHEM Trust calls for the next round of EU decision-makers to introduce policies that ensure we have toxic-free toothpastes and consumer products (e.g, withoug titanium titane, without endocrine disruptors, etc.)
- 23 March 2024 : Le Moniteur des pharmacies n°3506 asks: “Titanium dioxide and medicines, all white or all black?” and relays the opinions of experts from INRAé, LEEM and C2DS, as well as the insights of Mathilde Detcheverry from AVICENN and her key proposal: that health authorities make available to the public a regularly updated list of medicines available without E171, so that patients are informed of titanium dioxide-free references when they exist.
- 14 March 2024: The Danik consumer group Forbrugerrådet Tænk has identified 1️⃣1️⃣ toothpastes, out of the 3️⃣1️⃣ examined, that contain titanium dioxide (CI77891). It’s less than the year before.
- 29 February 2024 : A study published in Frontiers in Pediatrics and carried out by researchers from Bordeaux shows that in utero exposure to E171 and its titanium dioxide nanoparticles can impair respiratory function in newborn mice.
- 23 Januarty 2024 : A study published in Discover Nanoshows that in utero exposure to E171 and its titanium dioxide nanoparticles causes lung problems in mice, with repercussions for several generations.
- The January 17 issue of L’information dentaire publishes an article entitled ‘Nanomaterials in the dental office. Identify them to promote health’, which ends with an insert dedicated to titanium dioxide nanoparticles in toothpastes and medicines: “TiO2: towards an acceleration of its removal thanks to awareness-raising work!”
- 1 January 2024 : Prescrire’s article Bad in food, good in health products? questions the continued use in health products of Bisphenol A and titanium dioxide (E171), two substances known to be harmful in food.
- January 2024 : The AVICENN nano-chronicle published in issue 528 of S!lence magazine asks the question: E171 in medicines: indispensable, really?
- January 2024 : Publication of the repost “Should titanium dioxide be banned from medicines?” by master’s students from the École des Mines de Paris – Mines Paris PSL (as part of the “Description of controversies” course):

In 2023
- 5 & 8 December 2023 : On December 5, the European Committee for Consumer Safety (SCCS) publishes its preliminary scientific opinion on TiO2 in cosmetics, available for comment until February 6, 2024. Interviewed by ENDS Europe, Natacha Cingotti, from HEAL, commented asking for the implementation of the precautionary principle: “the European Commission should not allow it in cosmetics products as long as the important health concerns cannot be excluded”.
- 23 November 24 2023: In an article entitled “Nanoparticles: why so many drugs still contain titanium dioxide“, the OBS recalls that E171 “is used to whiten tablets and stabilize formulas. “It protects tablets from ultraviolet radiation and thermal conditions which could disrupt the stability of a product”, explains Thomas Borel of Leem (the French federation of medicine companies). An argument often dismissed by the scientific community which considers that medicines enclosed in opaque boxes and often in blisters are, in general, well protected from solar radiation”, writes Christelle Pangrazzi, who adds: “what about its effects in patients suffering from chronic pathologies requiring several medications, daily, for months, even years?”
- 23 November 2023: An article by researchers from INSERM, CEA, CNRS, CEA, etc. published in Particle and Fibre Toxicology shows that perinatal foodborne titanium dioxide exposure-mediated dysbiosis predisposes mice to develop colitis through life.
- 22 August 2023 : 60 millions de consommateurs warns about the presence of titanium dioxide (CI 77891) in lipsticks : “All the lipsticks in our selection contain it”, although it is “suspected of promoting colorectal cancer”.
- 20 July 2023 : In its article entitled “Towards a total ban on titanium dioxide?“, 60 millions de consommateurs magazine points out that the situation is moving more slowly on the drugs front than on the toothpaste front. Citing figures provided by AVICENN, the magazine points out that TiO2 has been withdrawn from almost 75 toothpaste references since 2019, not counting the very many new toothpastes that have since been formulated without TiO2 as soon as they were first placed on the market. On the drugs side, however, references are rarer for the time being. But the trend towards elimination should also accelerate: “Upsa has withdrawn TiO2 from Efferalgan film-coated tablets and Sanofi (…) from all Doliprane formulations, except capsules”.
- 18 July 2023: AVICENN publishes the results of its survey on VeilleNanos: in France, titanium dioxide (TiO2) has been removed from around 50 toothpastes in 2 years. While some brands are lagging behind, most have begun to remove it from their toothpastes, and some have even banned it altogether. Our results were reported in several media (Reporterre, Fémina, 60 millions de consommateurs, …)
- May-June-July 2023 : Published within a few days of each other, two articles from INRAE warn about chronic oral exposure to titanium dioxide: not only is E171 likely to promote certain metabolic disorders leading to diseases such as diabetes or obesity, but its nanoparticles can pass directly into the bloodstream via the mucous membranes of the mouth, and damage the DNA of exposed human oral cells.
After VeilleNanos on May 17, other media have reported the results of this research France info and Femina on May 19, Le Quotidien du Pharmacien and CosmeticOBS on May 22, Reporterre and Que Choisir on May 23, Ca m’intéresse on May 24, Pourquoi Docteur on May 25 and Marie Claire on May 29, Marie France on June 5, dentaire 365 on June 6, Le Mag de la Santé (France 5) on June 7, La Dépêche on June 28, Passeport Santé on July 3..…
In 2022
- December 18, 2022: Following the publication of our report In search of nanos in everyday products reporting the presence of titanium dioxide nanoparticles in the tested drug, AVICENN received this email from Alain J, reproduced with his permission:
“Treated for heart problems, I take atenolol (Tenormin), amlodipine, and atorvastatin for cholesterol: these 3 drugs contain E171 as an excipient. Still for the heart, I take Kardegic 75 which causes weakness of the blood vessels, hence hemorrhoids which I treat with Daflon, also containing E171.
Unfortunately, I suffer from prostatitis, so I take either Permixon or Tadenan, 2 pills a day, also containing E171…
When I have trouble sleeping, I take Zopiclone, which contains the same excipient, and if I want to make it lighter by taking Euphytose, I find myself facing the same problem…(…)
In short, I find that the cumulative effect is not taken seriously either by the laboratories or by the Ministry of Health, while pastry chefs have excluded this product from their preparations. (…) There you go, just in case my voice could be heard somewhat, and you could help with that.”
- December 14, 2022: Anses published its opinion on the risk assessment of the nanometric fraction of the food additive E171 which points out the lack of toxicological data available to perform a complete assessment of the additive E171 and recommends limiting the uses and exposures of workers and consumers to nanomaterials, “by promoting the use of safe products, free of manufactured nanomaterials, and by limiting these uses to those considered in fine as duly justified and subject to a documented demonstration of risk acceptability”.
- June 21, 2022: The European Commission asked the Scientific Committee on Consumer Safety (SCCS) to re-evaluate the safety of TiO2 in cosmetics, with regard to its genotoxicity in case of inhalation and oral exposure. Among the types of cosmetics mentioned: lip balms, lipsticks, toothpastes, powders and hair sprays. The CSSC has nine months to issue its opinion, which should therefore be finalized in March 2023.
- January 14, 2022: the European regulation 2022/63 banning the food additive titanium dioxide (E171) opens the possibility, in its article 3, of a ban on E171 in medicines – a decision that could be taken in 2025. By April 2024, the European Medicines Agency (EMA) will need to complete an updated assessment before the European Commission can reconsider the need to keep titanium dioxide (E171) on the list of colorants used in medicines, taking into account progress made in the meantime to develop alternatives. “If the replacement of titanium dioxide (E171) in medicinal products has not taken place or has not started within the above-mentioned time frame, only verifiable objective reasons related to the impossibility to replace it should be taken into consideration”
In 2021
- December 31, 2021: relaying the publication in the Official Journal of the renewal of the suspension of E171 in France for one year, the Midi libre headline read:“Food: titanium dioxide banned from French food products but still present in medicines“
- December 24, 2021: relaying the investigation of Kali (see above), France 3 interviewed Pauline Cervan of Générations futures who denounces the fact that titanium dioxide has no role on the effectiveness of drugs, but presents risks of carcinogenicity and genotoxicity
- December 22, 2021: Kali magazine made its “front page” on the 800 drugs containing titanium dioxide and calls for the withdrawal of TiO2 from drugs

- October 28, 2021: Que Choisir denounced, with AVICENN, the fact that the European Medicines Agency (EMA) is the voice of laboratories and regretted that a deadline for substitution, provided by a regulatory text, had not been established by the European Commission
- October 21, 2021: Karine Jacquemart of Foodwatch called for a ban on titanium dioxide in medicines and toothpaste.
- October 2021: following the approval by the Member States of the ban on E171 in food throughout the European Union, we learned that medicines will not be affected for several years
- May 6, 2021: the European Commissioner for Health and Food Safety, Stella Kyriakides, announced that the European Commission will propose a European ban on E171; this announcement was made just hours after the publication of the notice of the European Food Safety Agency (EFSA) concluding that this additive can no longer be considered “safe”, due to potential genotoxic effects (DNA damage). This is a clear shift in EFSA’s position, who until now had been adamant that E171 is safe – despite the numerous scientific publications that have been accumulating for several years and showing adverse effects. This reversal confirms the relevance of the alerts launched – for more than ten years now – by scientists and associations and taken seriously by the French authorities, who have suspended E171 since 2020.
- March 4, 2021: the association Agir pour l’Environnement announces on Facebook that 7 new brands have committed to remove titanium dioxide from their toothpastes; their website https://dentifrice-infoconso.agirpourlenvironnement.org has recently been updated
In 2020
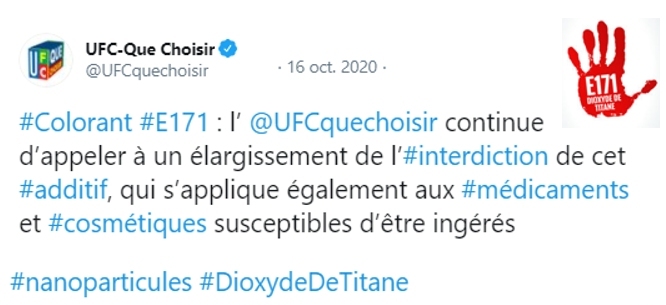
- October 22, 2020: the association Agir pour l’Environnement launched a petition “Stop titanium” asking for the extension of the ban on titanium dioxide to medicines and toothpastes (more than 30 000 signatures collected):

- 16 October 2020: Que Choisir also reiterated its call for a broadening of the ban on this additive to drugs and cosmetics likely to be ingested (toothpaste, lipstick …):
- October 7, 2020: a study by French researchers showed that pregnant women’s exposure to titanium dioxide leads to an accumulation of TiO2 nanoparticles in the placenta and contamination of the fetus. It was conducted by scientists from INRAE, LNE, the Rouen Materials Physics Group, the Toulouse University Hospital, the University of Picardie Jules Verne and the Toulouse National Veterinary School. It confirmed strong presumptions, following publications of tests in animals. As the INRAE press release reminded us, the use of titanium dioxide in foodstuffs has been suspended in France, but it is still used in toothpastes, anti-UV screens, cosmetic creams and powders and pharmaceutical products. In a press release published the same day, Agir pour l’Environnement asked for the extension of the E171 ban to medicines and toothpastes.
In 2019
- 19 November 2019 : NGOs Agir pour l’Environnement and AVICENN, interviewed on the Swiss consumer reference program “A bon entendeur” mention the impact of TiO2 ban in food on the toothpaste and pharmaceutical sectors (“domino effect”)
- August 2019: the LR senator of the Alpes-Maritimes, Colette Giudicelli, submitted a written question (No. 11991) to the Minister of Solidarity and Health at the time, on the presence of nanoparticulate titanium dioxide in toothpastes and some drugs. At the end of September, AVICENN learned that the question, although forwarded to the Ministry of Economy, Finance and Recovery, has been “withdrawn due to the death of the senator”.
- June 2019: the consumer association Que Choisir urged the European authorities to ban without delay the titanium dioxide present in nearly 7000 cosmetic references likely to be ingested (toothpastes, mouthwashes, lipsticks and lip balms, etc.).
- June 2019: Interviewed by Challenges7Cf. Why titanium dioxide has been banned from plates, but not from toothpastes, Challenges, June 7, 2019, Anne Dux, director of scientific affairs at Febea, the professional association of the cosmetics sector, apparently answered that in toothpastes, there is no possible substitute for TiO2 as a white colorant because titanium dioxide is the only one not to interact with other elements. The obstacle to its removal according to her: “studies show that in the minds of consumers, white is associated with cleanliness and that this encourages them to brush their teeth more”. However, some brands have always done without it, others have already started to remove it from their toothpastes and some even make the absence of TiO2 in their toothpaste a marketing argument8In October 2018, the retailer Casino, pledged to remove TiO2 nanoparticles from “all of its products,” including toothpaste, by the end of the year. In January 2019, AVICENN also spotted the Dentavie brand toothpaste marketed by Laboratoire Léa Nature, which is guaranteed to be “free of unnecessary and controversial ingredients,” including no “titanium dioxide.”.
- March 2019: the association Acting for the Environment (APE) asked the cabinet of the Minister of Economy and Finance, Bruno Le Maire, to extend the suspension of titanium dioxide to all products that can be totally or partially ingested: toothpastes and medications in particular, reporting the presence of titanium dioxide (TiO2) in two thirds of the 408 toothpastes. Not one was labeled [nano], mention mandatory on the packaging for any ingredient of nanometric dimension present in cosmetics. The day after the meeting, Agir pour l’Environnement indicated in a press release that “the meeting was very disappointing”, because there was no question, for the moment, of extending the scope of the decree to products other than food for various reasons. One of the reasons mentioned was that the draft order was designed for a food framework…but this framework could be changed in the drafting of the order. On the absence of [nano] labeling of titanium dioxide, the DGCCRF had not yet conducted investigations on toothpastes but indicated that it had planned to do so in the coming months. For Agir pour l’Environnement, this status quo showed “once again” the timidity of the Ministry of Economy, unable to protect consumers, children and patients, from exposure to a dangerous and unnecessary chemical substance in food, toothpaste and medicines. Without waiting for an investigation by the DGCCRF and the dissuasive sanctions that Agir pour l’Environnement was calling for, it put the toothpaste.infoconso. org website online. The site allows to quickly identify toothpastes with and without titanium dioxide. In the Agir pour l’Environnement investigation report published at the time, the association indicated that it had found TiO2 in two-thirds of the 408 toothpaste compositions it studied (including 25 organic toothpastes) and in half of the 60 children’s toothpastes. It also revealed that none of the 271 toothpastes concerned bore the mention [nano], which is mandatory on the packaging for any ingredient of nanometric dimension present in cosmetics. Agir pour l’environnement indicated that it had also launched a participatory survey on the presence of titanium dioxide in medicines.
In 2018
- July 2018: according to the Ministry of Solidarity and Health, ANSM referred to the European Medicines Agency (EMA) about excipients in nanometric form entering the composition of authorized medicines.
- October 2018: “60 Millions de consommateurs” denounced the almost systematic use of TiO2 for applications where the anti-UV function is not strictly necessary is controversial, notably in anti-wrinkle creams: “The filters incorporated in these anti-wrinkle products are controversial. In particular (…) titanium dioxide in nano form. In a care cream with a purely aesthetic aim such as an anti-wrinkle cream, the presence of UV filters with a proven risk, or even only suspected of toxicity, is not acceptable”.9Cf. ” Anti-wrinkle creams: unwelcome UV filters,” 60 Millions de consommateurs, October 25, 2018.
- June 2018: online petition “Stop carcinogenic titanium dioxide in our medicines!” (to the attention of the Minister of Health, on the platform citizaction.fr which is no longer online) > early November 2019 it had collected more than 20,000 signatures
- February 2018: Que Choisir highlighted TiO2 in Aquafresh toothpaste with 40% of particles smaller than 100 nm.
In 2017
- October 2017: BD nano waq published, co-financed by Agir pour l’Environnement, Générations futures, France Nature Environnement and the Committee for Sustainable Development in Health (C2DS)
- June 2017: “Temporarily banning nanomaterials of questionable safety in food, drugs and toothpaste in France” is one of the eleven proposals compiled by AVICENN, in partnership with its associated members and other civil society actors as well as risk assessment and management bodies in the framework of the nanomaterials labeling/restriction working group led by the Ministry of the Environment
- February 4, 2017: Que Choisir revealed that it has counted 4,000 medicines containing TiO2
In 2009
- During the national public debate on nanotechnologies, two papers addressed this subject:
Any questions or comments? This information sheet compiled by AVICENN is intended to be completed and updated. Please feel free to contribute.
Our information sheets to go further
Upcoming Nano Agenda

- International conference on metallic nano-objects for experts working in the interdisciplinary field of metallic nanoparticles, with a particular emphasis on nanoparticle synthesis and characterization, plasmonics, optics and photonics, catalysis, biomedicine, electronics, and nanoparticle recycling
- Organizers / Partners: CNRS, Bordeaux University, Bordeaux INP, ICMCB, CRPP, CBMN, ISM
- Website: https://mno2026.sciencesconf.org

- Seminar presenting the Seirich system for chemical risk assessment, with a focus on the specificities of nanomaterials.
- Organizers: Association NanoMesureFrance
- Speakers: Florian Marc (INRS)
- Website: www.eventbrite.fr/e/billets-nanomesurefrance-webinar-1984136502691
- Toxicokinetics: the fate of chemicals in the body” training course:
- the different routes of entry for toxic products
- the importance of toxicokinetics in preventing substance toxicity,
- xenobiotic absorption, distribution, metabolism and elimination
- nanoparticle toxicity
- Organizer: Association Toxicologie Chimie (ATC)
- Date: March 26, 2026
- Speaker: Nicole Proust (Research Engineer, CNRS Honorary Research Director, Palaiseau)
- Website: www.atctoxicologie.fr/…
File originally created in March 2019
Notes and references
- 1smaller than 100 nanometers (1 nm = 0.001 µm = 1 billionth of a meter)
- 2See recitals 14 to 18 and article 3 of the European regulation 2022/63.
- 3Cf. Authorization of E171 in medicines “should be maintained”, at the request of pharmaceutical companies, AVICENN @VeilleNanos, 14 août 2025
- 4More details in the Minutes of the WG on Nanomaterials in Cosmetic Products, SCCS, 7 and 25 April 2025
- 5(82 our of 84)
- 6(RM09 and RM11, for which the provided genotoxicity data indicate no genotoxicity concern)
- 7Cf. Why titanium dioxide has been banned from plates, but not from toothpastes, Challenges, June 7, 2019
- 8In October 2018, the retailer Casino, pledged to remove TiO2 nanoparticles from “all of its products,” including toothpaste, by the end of the year. In January 2019, AVICENN also spotted the Dentavie brand toothpaste marketed by Laboratoire Léa Nature, which is guaranteed to be “free of unnecessary and controversial ingredients,” including no “titanium dioxide.”
- 9Cf. ” Anti-wrinkle creams: unwelcome UV filters,” 60 Millions de consommateurs, October 25, 2018