
TiO2 in cosmetics: according to European experts, safety data are inconclusive
The highly detailed scientific opinion of the European Scientific Committee on Consumer Safety (SCCS) published at the end of May is clear: the data provided by manufacturers are not robust enough to remove doubts about the potential genotoxicity of titanium dioxide (TiO2) in cosmetics. The SCCS is calling for more experimental data, especially on toothpastes, since consumers use them every day. The European Commission has granted manufacturers additional time to gather better quality data. In the meantime, European consumers and workers continue to be exposed to various types of TiO2s, with no guarantee of their harmlessness and against the precautionary principle.
Background: an opinion requested by the European Commission two years ago
Two years ago, in June 2022, the European Commission asked the Scientific Committee on Consumer Safety (SCCS) to reassess the safety of titanium dioxide (TiO2) in cosmetics. This request followed the receipt by the European Commission, the month before, of a dossier sent by the cosmetics industry to demonstrate the safety of TiO2 in cosmetics. This colorant had been banned from foodstuffs since January 2022, following the publication in May 2021 of the opinion of the European Food Safety Authority (EFSA) reporting doubts on the safety of the food additive E171 (composed of TiO2) because of a potential genotoxicity.
The Commission had given the SCCS nine months to deliver its opinion, which was due in March 2023. However, as the Cosmetics Europe trade association was slow to provide the additional data requested by the SCCS, the latter was unable to publish its draft opinion until December 2023, when it was submitted for consultation. The final opinion was adopted and published in May 2024.
In over 97% of TiO2 forms supplied by industry, the risk of genotoxicity cannot be ruled out
In particular, the expert committee had to focus on genotoxicity
and exposure via the inhalation and oral route (lip care, lipstick, toothpaste, loose powder, hair spray).
In the final version of its scientific opinion #1661/23, the SCCS considers that the available evidence is not sufficient to exclude the genotoxicity potential of almost all of the types of TiO2 forms used in oral cosmetic products – both for the 44 “pigmentary” TiO2s (presented as non-nano*), and for 38 of the 40 “nano” TiO2.
→ Only two nano TiO2 nanos were displaying data indicating no genotoxicity concern, but the SCCS also adds that “more information is, however, needed on their potential uptake and cellular effects in the oral mucosa to consider them safe for use in oral-care products” (knowing that the reader being can not identify in which products they are used):
- RM09: its nanoparticles have a mean size of 26 nm, are hydrophilic and coated with amorphous silica;
- RM11: its nanoparticles have a mean size of 20 nm, are hydrophobic and coated with alumina.
For the other 82 TiO2s, the data presented by the cosmetics industry to prove the absence of genotoxicity were deemed inconclusive by the SCCS, due to various pitfalls:
- insufficient description of the chosen methodology,
- no reference to the protocol for dispersion used for sample preparation,
- no proof of cell internalisation by TiO2 particles,
- limited number of animals used,
- too long a delay between exposure of the animals and analysis of their DNA,
- tests executed on bronchoalveolar lavage fluid analysis instead of lung tissue,
- no confirmation by transmission electron microscopy,
- tests carried out on TiO2 forms which are mainly not used in cosmetics, and therefore not relevant to this evaluation
- …
The scientific literature does not rule out the risk of genotoxicity of TiO2 in cosmetics either
To complete its assessment, the SCCS also carried out an analysis of the scientific literature: from 353 pre-selected articles, the SCCS selected 284 as being sufficiently relevant, detailed or scientifically robust. Its conclusions: in view of the data currently available and the insufficient weight of evidence, the safe use of TiO2 in cosmetics can not be confirmed:
- nor for the various types of pigmentary TiO2s (the SCCS agrees with the EFSA opinion, which focused on TiO2 used in foodstuffs, E171), for which the risk of genotoxicity cannot be ruled out;
- nor for nano TiO2s, made of coated rutile, for which the risk of genotoxicity has not been determined.
European experts call for further investigations, with even greater focus on toothpastes
The SCCS therefore considers that more experimental data are needed to better assess and, if necessary, exclude the genotoxicity potential of the various TiO2s (both pigmentary and nanos) used in oral cosmetic products. He suggests that additional tests be carried out for this purpose (including in vitro micronucleus or chromosome aberration tests, conducted in accordance with state-of-the-art nanotoxicology principles).
The SCCS also adds that, given that the cells of the oral mucosa are likely to absorb TiO2 nanoparticles and that some oral products containing TiO2 nanoparticles, such as toothpastes, are used every day and potentially more than once a day, further investigations are needed: without them, the risk linked to long-term repeated exposures of the biccal mucosa to TiO2 nanoparticles cannot be excluded.
Despite inconclusive data, industry gains two more years before potential European restrictions
What will be the regulatory consequences of this opinion? Is the European Commission planning to take measures to restrict the use of TiO2 in cosmetics whose safety is not guaranteed? In response to our question sent to the Commission, we learned that, following the SCCS opinion, the cosmetics industry committed “to provide more data allowing the SCCS to conclude on a safe use of TiO2 in oral applications“. The Commission, with the support of the Member States, agreed to give the industry time until the end of 2024 to provide good quality data on which basis the SCCS would be able to conclude its assessment. The Commission will reassess the situation at the beginning of 20251Coincidentally, this is also when the Commission will have to decide on a ban on TiO2 in medicines (February 2025 at the latest), on the basis of the report submitted by the European Medicines Agency (EMA) in April – a report which has not been made public yet….
The European Commission is therefore giving the benefit of the doubt to the cosmetics industry, which will thus gain at least two more years (between the beginning of 2023 – the date initially expected for the SCCS to deliver its opinion, and the beginning of 2025 – the date of the Commission’s decision). Two years during which their products containing TiO2 will have been marketed, with no guarantee of the substance’s safety…
In view of the adverse effects of TiO2 highlighted in other publications2(not only genotoxic, but also reprotoxic, neurotoxic, respiratory, behavioral effetcs, etc.). Cf. our articles and our fact sheet on the risks associated with TiO2 nanoparticles), isn’t the Commission being too conciliatory with the cosmetics industry, betting that by the end of 2024 it will provide irrefutable proof of the absence of TiO2 genotoxicity – despite the flippant way in which the representatives of cosmetics industry interests and titanium dioxide manufacturers have behaved to date?
→ Remember that in 2014, TiO2 manufacturers opposed requests from the European Chemicals Agency (ECHA) which had asked for information on the TiO2 nanoforms they were manufacturing (on the grounds that the term “nanoform” and “grade” (in English) were not sufficiently well defined according to the manufacturers)!
Since then, TiO2 manufacturers lobbying has continued (not only in the field of cosmetics, but also in that of pharmaceuticals and with regard to classification as a category 2 carcinogen by inhalation), as documented by various NGOs (including Corporate Europe Observatory (CEO), the European Environmental Bureau (EEB), Health & Environment Alliance (HEAL), AVICENN3 See our news item of November 23, 2022 and our fact sheet on the risks associated with TiO2 nanoparticles, …).
This new delay is yet another illustration of the effectiveness of chemical industry lobbying, whose tricks – as big as they are effective – are deciphered by the NGO ChemSec in a post relayed yesterday online4Five hocking truths about #EU chemicals legislation that will make you rethink everything you thought you knew about safety regulations, ChemSec, July 2024 → Harmful chemicals in a nutshell, ChemSec, 2024 : “chemical producers can block the chemical bans for decades”(…) by “deliberately slowing down the regulatory process to keep their chemicals on the market for as long as possible”. In fact, “in many cases, the outcome of experts groups commissioned by European institutions is often requests from the industry for more data to clarify concerns rather than immediate regulation. All the while, it is perfectly legal to use these harmful chemicals in consumer products.When it’s then determined that more data is needed, industries are given several years to comply“.
Doesn’t consumer safety deserve better? Given the high tonnages of TiO2 used in cosmetics, fully independent studies, conducted according to good laboratory practices, should have been launched long ago to provide reliable data on the safety of the various forms of this substance, especiallyof its nanoparticulate components.
The precautionary principle, forgotten by public authorities but applied by a growing number of brands
The European NGO HEAL considers that “the genotoxicity risks of TiO2 should have led the European Commission to apply the precautionary principle; TiO2 should no longer be authorized until proven safe, because if it damages DNA, there is a risk of cancer in the long run”.
The irony in this case is that the precautionary principle is not being applied by the European Commission… but it is applied by some companies, who have taken the initiative to remove TiO2 from their cosmetics, starting with toothpastes – even though they are not legally obliged to do so (at least not yet).
By this time last year, AVICENN had identified a drastic reduction in the number of toothpaste references containing TiO2 in France since 2019: TiO2 (indicated with the code CI77891) has been removed from 75 toothpaste references, and new toothpastes formulated since 2019 are, for the most part, TiO2-free from the moment they are first placed on the market.
The risks associated with TiO2 are also now receiving more attention outside France. As with the ban on TiO2 in food, which was first anticipated by certain French brands before being adopted by French and then by European authorities, this concern is spreading beyond our borders.5In March this year, the Danish consumer association Forbrugerrådet Tænk counted 11 toothpaste references, out of 31 studied, containing titanium dioxide, i.e. fewer than last year. And a few months later, in June, a survey conducted by Great Italian Food Trade (GIFT) in Italy’s supermarkets and pharmacies set out to investigate the presence of titanium dioxide in toothpastes sold in Italian.
We need to protect not only consumers, but also workers.
ANSES, which has taken over from ANSM the task of monitoring and assessing cosmetic products, said it submitted a comment as part of the consultation on its draft opinion launched by the SCCS in December 2023. AVICENN has expressed its wish for their comment to be made public, and ANSES is studying the possibility of publishing it “by the end of the year”. ANSES is also conducting other work on the risks associated with TiO2 handled in the workplace6ANSES’s work on TiO2 is feeding the work of the French authorities’ appeal to the Court of Justice of the European Union (CJEU) concerning the classification of TiO2 as a suspected carcinogen, as well as the development of an occupational exposure limit value (OELV) for nano TiO2, the evaluation of methods for measuring TiO2 in air, and the recommendation of a measurement method for worker exposure. It’s a reminder that it’s not just consumers who need to be protected from the risks associated with inhalation of TiO2 nanoparticles, but also workers.

* Pigmentary TiO2 vs. nano TiO2 – an artificial and debatable distinction
* AVICENN would like to come back to the distinction made by the cosmetics industry, as relayed by the SCCS, between:
- pigmentary” TiO2 presented as non-nano** with median primary particle size > 100 nm, and whose primary function is to provide whiteness and opacity as well as a some UV protection
- nano” TiO2 with median primary particle size <100 nm, whose primary function is to provide UV attenuation without excessive whiteness.
** Although presented as “non-nano”, some of the TiO2s presented as “pigmentary” by the industry may contain a nanoparticulate fraction flirting with 50%, making them very close to so-called “nano-TiO2s”.
It’s important to understand that this distinction between “pigmentary” TiO2s on the one hand, and “nanosized” TiO2s on the other, is based on the European Commission’s 2011 and 2022 recommendations for the definition of the term “nanomaterials”, which have introduced a threshold of 50% of particles smaller than 100 nm – which does not appear in the Cosmetics Regulations in force since 2009. Of course, this regulation is due to be revised, and the Commission and the cosmetics industry share the desire to include the 50% threshold. But as time goes by, this prospect recedes ever further, to the point where even the cosmetics industry is beginning to doubt whether it will ever really see the light of day.
Yet this distinction raises questions: not only are the differences in properties relatively tenuous and absolutely unquantified (on the pigment TiO2 side: “some UV protection”, on the nano TiO2 side: UV “attenuation” “without excessive whiteness”), but questions of size are also debatable:
- First, some TiO2s have been classified as such by the cosmetics industry on the basis of a scanning electron microscopy (SEM) measurement giving a result below the 50% threshold, whereas a transmission electron microscopy (TEM) measurement gives a result above 50% (up to 66.7%), which should lead to them being considered as nanos.
- Also, the smallest median sizes of the constituent particles of pigmentary TiO2 is just above the 100 nm limit by SEM/SEM (103 nm), but well below (85 nm) by TEM/TEM :
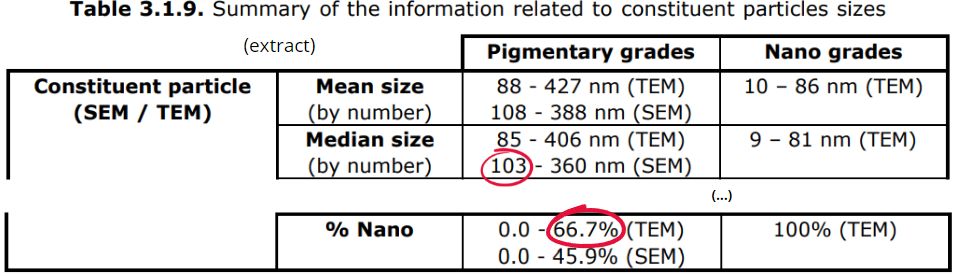
Source: Scientific Advice on Titanium dioxide (TiO2), SCCS, 1661/23, May 2024 (page 32)
→ These metrological subtleties and the disparities in the data and interpretations made of them concretely illustrate and question the artificial, not to say arbitrary, dimension of the 50% threshold of particles smaller than 100 nm that the European Commission and the cosmetics industry wish to impose in the regulations – and this, despite the opposition of other players (including the European Parliament, ANSES, the French authorities and NGOs) which led to the rejection of its inclusion in the novel foods regulations three months ago.
Eighteen months ago, AVICENN pointed out in its 2022 survey report that the cosmetics industry was lobbying to have the recommended definition of nanomaterials with the 50% threshold incorporated into European regulations , which favors corporate interests at the expense of consumer information and protection. Because in the end, brands that have been playing the wait-and-see card, by not complying with the [nano] labeling obligation since it came into force in 2013, have gained more than a decade during which they used unauthorized nanos, without labeling them [nano].
What about the TiO2 contained in the pearlescent pigments in vogue in the make-up department?
What about the risks associated with the pearlescent pigments in vogue in the make-up department? They are often composed of TiO2 nanoparticles that are not authorized as nanoscale colorants, and the question of their safety arises all the more as they are likely to be inhaled and are present in a growing number of make-up products: eyeshadows, blushes and face powders7(the L’Oréal powder tested by AVICENN in 2022, for example, was composed of TiO2 nanoparticles – with 100% of particles smaller than 100 nm and a median diameter of around 50 nm (Cf. Searching for [nanos] in everyday products, AVICENN, December 2022); AVICENN has questioned the French control authorities (DGCCRF) on this subject, which claims that their investigations are confidential).
→ The SCCS opinion does not mention these pearlescent pigments, and the previous opinion, published in 2020, had explicitly ruled them out, due to the composite nature of these pigments. In fact, the TiO2 nanoparticles that give makeup its iridescent appearance are attached to mica plates. As such, the European Commission is not willing to consider them as nanomaterials anymore8Cf. recital 11 of its June 2022 definition recommendation (and therefore they wouldn’t be regulated them as such under the future Cosmetics Regulation).
However, the presence of this very high proportion of metal oxide nanoparticles in this type of make-up raises safety issues, and a scientific opinion from the SCCS would therefore be very useful. Indeed, the recent restriction on microplastics 2023/2055 adopted by the European Commission last September, some of which were used as glitter in cosmetics, will most likely lead to a substitution by – and therefore an increase in – mica coated with TiO2 nanoparticles in order to maintain the iridescent effect of make-up9See for example: https://www.linkedin.com/posts/maprecos_lesynthaeztiquecestchic-mica-paillette-activity-7140274133967237120-7EgY/ and https://www.alexmo-cosmetics.de/Pearl-effect-pigments. It is therefore urgent for the European Commission to ask the SCCS to carry out a risk assessment of these pearlescent pigments, without waiting for years.
Other nano-related news
Next nano events
- Introductory seminar on the principles of Life Cycle Assessment (LCA) and its specificities when applied to nanomaterials (metal nanoparticles, oxides, etc.), from their production to their end-of-life.
- Organizer: NaMasTE research group (Manufactured Nanomaterials, Toxicology, Ecotoxicology and Risks: Towards Controlled Development)
- Speaker: Gaetana (Tania) Quaranta, Senior Lecturer, University of Strasbourg – IPHC
- Website: https://gdr-namaste.cnrs.fr/

- Webconference for analysis laboratories, plant fertilizer manufacturers and distributors, public authorities…
- Moderated by David Krupka, nanotechnologies development manager at AFNOR Normalisation and Emilie Langlois-Bertrand, nantechnologies standardization project manager.
- In partnership with Armand Masion (CEREGE) and Patrice Charpentier (ANSES).
- This exchange will also be an opportunity to explore the creation of a national platform to identify standardization needs.
- Website: https://www.afnor.org/evenements/qualite/nanotechnologies-agriculture-cadre-pratique-responsable

- International conference on metallic nano-objects for experts working in the interdisciplinary field of metallic nanoparticles, with a particular emphasis on nanoparticle synthesis and characterization, plasmonics, optics and photonics, catalysis, biomedicine, electronics, and nanoparticle recycling
- Organizers / Partners: CNRS, Bordeaux University, Bordeaux INP, ICMCB, CRPP, CBMN, ISM
- Website: https://mno2026.sciencesconf.org
Notes and references
- 1Coincidentally, this is also when the Commission will have to decide on a ban on TiO2 in medicines (February 2025 at the latest), on the basis of the report submitted by the European Medicines Agency (EMA) in April – a report which has not been made public yet…
- 2(not only genotoxic, but also reprotoxic, neurotoxic, respiratory, behavioral effetcs, etc.). Cf. our articles and our fact sheet on the risks associated with TiO2 nanoparticles)
- 3See our news item of November 23, 2022 and our fact sheet on the risks associated with TiO2 nanoparticles
- 4
- 5In March this year, the Danish consumer association Forbrugerrådet Tænk counted 11 toothpaste references, out of 31 studied, containing titanium dioxide, i.e. fewer than last year. And a few months later, in June, a survey conducted by Great Italian Food Trade (GIFT) in Italy’s supermarkets and pharmacies set out to investigate the presence of titanium dioxide in toothpastes sold in Italian
- 6ANSES’s work on TiO2 is feeding the work of the French authorities’ appeal to the Court of Justice of the European Union (CJEU) concerning the classification of TiO2 as a suspected carcinogen, as well as the development of an occupational exposure limit value (OELV) for nano TiO2, the evaluation of methods for measuring TiO2 in air, and the recommendation of a measurement method for worker exposure
- 7(the L’Oréal powder tested by AVICENN in 2022, for example, was composed of TiO2 nanoparticles – with 100% of particles smaller than 100 nm and a median diameter of around 50 nm (Cf. Searching for [nanos] in everyday products, AVICENN, December 2022); AVICENN has questioned the French control authorities (DGCCRF) on this subject, which claims that their investigations are confidential)
- 8Cf. recital 11 of its June 2022 definition recommendation
- 9



