
Revision of the definition of “nanomaterial” by the European Commission

Revision of the definition of the term “nanomaterial” by the European Commission (2014-2022)
By the AVICENN team – Last modification November 2024
The result of the revision of the recommendation for the definition of the term “nanomaterial” was published in June 2022; it marks the end of a long suspense (the revision was awaited since 2014…) and the beginning of a new adventure: the clarifications announced are not necessarily there and there is a good chance that quarrels on the qualification (or not) of certain substances as “nanomaterial” will continue…
The “revised” definition recommendation was published in June 2022
On June 10, 2022, the Commission published its revised recommendation for the definition of “nanomaterial,” which replaces its previous 2011 recommendation. It reads as follows:
“Nanomaterial” means a natural, incidentally or manufactured material consisting of solid particles* that are present, either on their own or as identifiable constituent particles in aggregates** or agglomerates**, and where 50% or more of these particles, in the number-based size distribution fulfil at least one of the following condition:
(a) one or more external dimensions of the particle* are in the size range 1 nm to 100 nm
(b) the particle* has an elongated shape, such as a rod, fiber or tube, where two external dimensions are smaller than 1 nm and the other dimension is larger than 100 nm
(c) the particle* has a plate-like shape, where one external dimension is smaller than 1 nm and the other dimensions are larger than 100 nm.
In the determination of the particle number-based size distribution, particles with at least two orthogonal external dimensions larger than 100 μm need not be considered.
However, a material with a specific surface area by volume of less than 6 m2/cm3 shall not be considered a nanomaterial.
* “particle” means a minute piece of matter with well-defined physical contours; single molecules are not considered “particles
** “aggregate”: means a particle comprising strongly bound or fused particles
*** “agglomerate”: means a collection of weakly bound particles or aggregates where the resulting external surface area is similar to the sum of the surface areas of the individual components.
An 8 year overdue, slow and tedious project
As a reminder, this review should have taken place in 2014… In 2011, the European Commission published a first recommendation for a definition that caused a lot of ink to flow. It was foreseen from the outset that this definition would, if necessary, evolve by 2014. In the end, after years of procrastination and postponements, this new definition was published mid-2022, eight years after the planned deadline.
At the 12th CASG nano meeting in March 2014, the European Commission had confirmed that this 2011 recommendation would be reviewed as planned.
In the same month, the Joint Research Centre (JRC) organized a technical workshop on this subject, then led a scientific discussion on the challenges of applying the definition in European regulations and published various reports and recommendations aimed at improving the content and implementation of the future definition1In 2014 and 2015 the JRC published an initial analysis report (See: Towards a review of the EC Recommendation for a definition of the term “nanomaterial” – Part 1: Compilation of information concerning the experience with the definition, JRC, March 2014), followed by an evaluation report (Towards a review of the EC Recommendation for a definition of the term “nanomaterial” – Part 2: Assessment of collected information concerning the experience with the definitionJRC, September 2014) and then a third report of recommendations to improve the content and implementation of the future definition (See Towards a review of the EC Recommendation for a definition of the term “nanomaterial”: Part 3: Scientific-technical evaluation of options to clarify the definition and to facilitate its implementation, JRC, July 2015).
The public consultation was postponed to 2015, then to 2016 and, in October 2020, after many twists and turns2In early May 2017, there were rumblings of an imminent release… and in early July, the trade press was talking about a summer release. In particular, the Commission confirmed, in mid-March 2017, before the Casg nano, that its draft revision would be subject to a 6-week public consultation after its publication, in order to be adopted in the second half of 2017 and accompanied by an explanatory note produced by the Joint Research Centre (JRC) attached to the Commission. A public and stakeholder consultation on the “roadmap ” did take place from mid-September to mid-October 2017, but it focused on the methodology, goals, and general principles guiding the development of the revised definition, more so than on the specific content of the new definition. 18 contributions were made, by industrialists, environmental agencies and some anonymous citizens. The roadmap mentions a 12-week consultation in the first quarter of 2018, but by the end of February 2019, feedback from Brussels indicated a more than likely postponement of the publication of the draft and the consultation to 2020 (see EU revision of nanomaterials definition postponed to 2020, Chemical watch, February 26, 2019), the European Commission reiterated its commitment to revise the definition of the term “nanomaterial” in its Chemicals Strategy for Sustainability ( CSS ) and to ensure its “consistent application across all legislation using legally binding mechanisms”. The annex specified a date in 2021…
On May 7, 2021, the European Commission did indeed finally publish its proposal and launched an online consultation, that was to end on June 30, 2021.
- AVICENN posted its contribution. It does not take a position on issues outside its field of competence, but rather fulfils its role as a watchdog, reminding us of the broader expectations of civil society for a better regulatory framework to ensure the protection of health and the environment.
- In a press release published on its website, the French National Health Safety Agency (Anses) summarized the content of its response to the European consultation on the definition of the term “nanomaterial”, without yet revealing its full content: Anses considered that “the modifications suggested by the Commission tend to restrict the number and nature of objects that will ultimately be considered as nanomaterials” and argued instead for the most inclusive definition possible, which does not exclude nanoplastics, emulsions and nanoscale lipids. It also underlined, as did AVICENN, the lack of scientific basis for the size thresholds (1-100 nm).
By mid-August 2021, the European Commission subsequently published the 136 contributions3Initially published on the following link https://ec.europa.eu/environment/pdf/chemicals/nanotech/TSC_Nanodefinition_PublicExcerpt.xlsx, they seem to have disappeared from the European Commission’s website; AVICENN had downloaded them in 2021 and has decided to upload them to veillenanos.fr in order to keep track of the positions defended at that stage by the stakeholders collected, along with additional contributions from the French authorities, the Industrial Minerals Association (IMA) and the German Federal Institute for Risk Assessment (BfR). At the time, the Commission indicated that it was conducting a “detailed analysis” with a view to publishing a summary and statistical analysis of the responses by the end of 2021, as well as its plans for the potential revision or replacement of the current definition recommendation.
In February 2022, the Commission once again failed to meet its commitments (there was still no synthesis or analysis of the contributions to the consultation, although they were promised for the end of 2021). Fifteen NGOs sent an open letter to the European Commission asking it to clarify and improve its definition of the term “nanomaterials” by opening the discussion so that this definition can be reworked collectively and then integrated – with or without adaptation – to the various European regulations.
Questions and controversies
As a reminder, the previous 2011 definition recommendation was challenged both by the industries who found it too wide and by NGOs who, on the contrary, considered it too restrictive as leaving out many nanometric materials.
The new 2022 recommendation is also a source of criticism (some are common to the 2011 definition, others are specific to the new 2022 version). The clarifications announced are not necessarily forthcoming4despite the details provided by the Commission staff working document (SWD(2022) 150), and it is likely that disputes will continue to arise over the qualification (or not) of certain substances as “nanomaterials. This is evidenced in particular by the reaction of the NGO CIEL published on June 14, 2022 and the Chemical Watch article relaying the dissatisfaction of CIEL and AVICENN on June 15, 2022. The reservations expressed by the ANSES on the preliminary draft submitted for consultation in 2021 (see below) had not been taken into account by the Commission either5Cf. Scientific and technical support note on “the development of a proposal for an updated definition of the term “nanomaterials” based on Recommendation 2011/696/EUAnses, January 14, 2022; Opinion and report on the request for scientific and technical support in drafting a proposal for an updated definition of the term “nanomaterial” based on European Commission Recommendation 2011/696/EU on the definition of nanomaterial, Anses, May 2023. Georges Favre, from the Laboratoire national de métrologie et d’essais (LNE), is less critical of this new definition 6Cf. La Commission publie sa nouvelle définition des nanomatériaux, Actu Environnement, 21 juillet 2022.
On May 17, 2023, Anses published an opinion on the new definition following a referral from the agency in 2018. Its conclusions are clear: the new definition of nanomaterials is detrimental to the prevention of health and environmental risks and public authorities must adopt a more inclusive definition.
At the end of 2023 – beginning of 2024, the European Commission attempted to implement, via a delegated act, its project to incorporate this 2022 “recommendation” into the Novel Foods Regulation, but this was rejected in April 2024 by the European Parliament.
In November 2024, reseachers from University of Bordeaux published an article in the Journal d’actualité du droit international et européen7Controverses juridiques sur la définition des nanomatériaux en droit européen, Marion Tissier-Raffin, Nickolas Achat, Didier Morin, Journal d’actualité du droit international et européen (JADIE), n°24, novembre 2024 also criticizing the fact that “the 2022 definition recommendation paves the way for a regression in the protection of public health and the prevention of health and environmental risks associated with nanomaterials, without providing solutions to the drawbacks of fragmented sectoral regulation“. They also deplore the fact that “the Commission gives precedence to industrial issues over health and environmental issues” and “(promotes) regulatory precaution rather than the precautionary principle, in the sense that the aim is clearly to limit as far as possible binding measures for market players in order not to hinder the market”.
What about above 100 nm?
Concerning the 1-100nm range (identical to 2011), there is no scientific justification to establish an upper and/or lower size limit to define all nanomaterials8See in particular:
– Nanomaterials: for a more protective European definition, Anses, 23 July 2021
– Contribution of AVICENN to the European consultation on the definition of the term “nanomaterial”, AVICENN, June 30th 2021
– Scientific Basis for the Definition of the Term “nanomaterial”, SCENIHR, European Commission, 2010. Results of toxicological studies show toxic effects specifically at the submicron scale above 100 nm, and specifically up to 600 nm scale. The American Food & Drug Administration (FDA) has chosen to define a nanomaterial as a material with at least one dimension smaller than 1000 nm9Cf. Reporting Format for Nanotechnology-Related Information in CMC Review, Office of Pharmaceutical Science (FDA), June 2010.
A team of Chinese and American researchers had published a paper on this topic in February 201410Bai Y et al, 100 Nanometers: A Potentially Inappropriate Threshold for Environmental and Ecological Effects of Nanoparticles, About. Sci. Technol., 48 (6) : 3098-3099, 2014.
What about internally structured nano materials?
In contrast to the ISO definition, the 2022 European definition recommendation, like the 2011 definition, excludes internally structured nano-materials11The International Organization for Standardization (ISO) defines a nanomaterial as “a material with any external dimension at the nanoscale or internal or surface structure at the nanoscale” (the nanoscale is defined as a spectrum of dimensions from about 1 nm to 100 nm); it has adopted the term NOAA to encompass all “Nano-Objects, their Agglomerates and Aggregates greater than 100 nm”.
Exclusions of different categories of materials
In addition to these grievances, there are additional questions about the new formulations adopted by the 2022 recommendation: for example, it does not take into account the questions and reservations of Anses, in particular12Scientific and technical support note on “the development of a proposal for an updated definition of the term “nanomaterials” based on Recommendation 2011/696/EU”, Anses, 14 January 2022 and could lead to the exclusion of nano-objects previously considered as nanomaterials:
- the introduction of the term “solid” excludes gaseous and liquid particles, in particular emulsions and micelles (the latter, used for medical applications, have an increased capacity to pass certain biological barriers)
- the exclusion of “single molecules” could exclude from the definition of nanoplastics, fullerenes and, again, nanomicelles and lipid structures used as vectors
- the exclusion of nano-composites could be used by some brands to escape regulatory constraints on the grounds that their ingredients are complex compounds, in which several substances are present (for example, titanium dioxide nanoparticles coated with silica or grafted onto mica plates, used in particular in cosmetics).
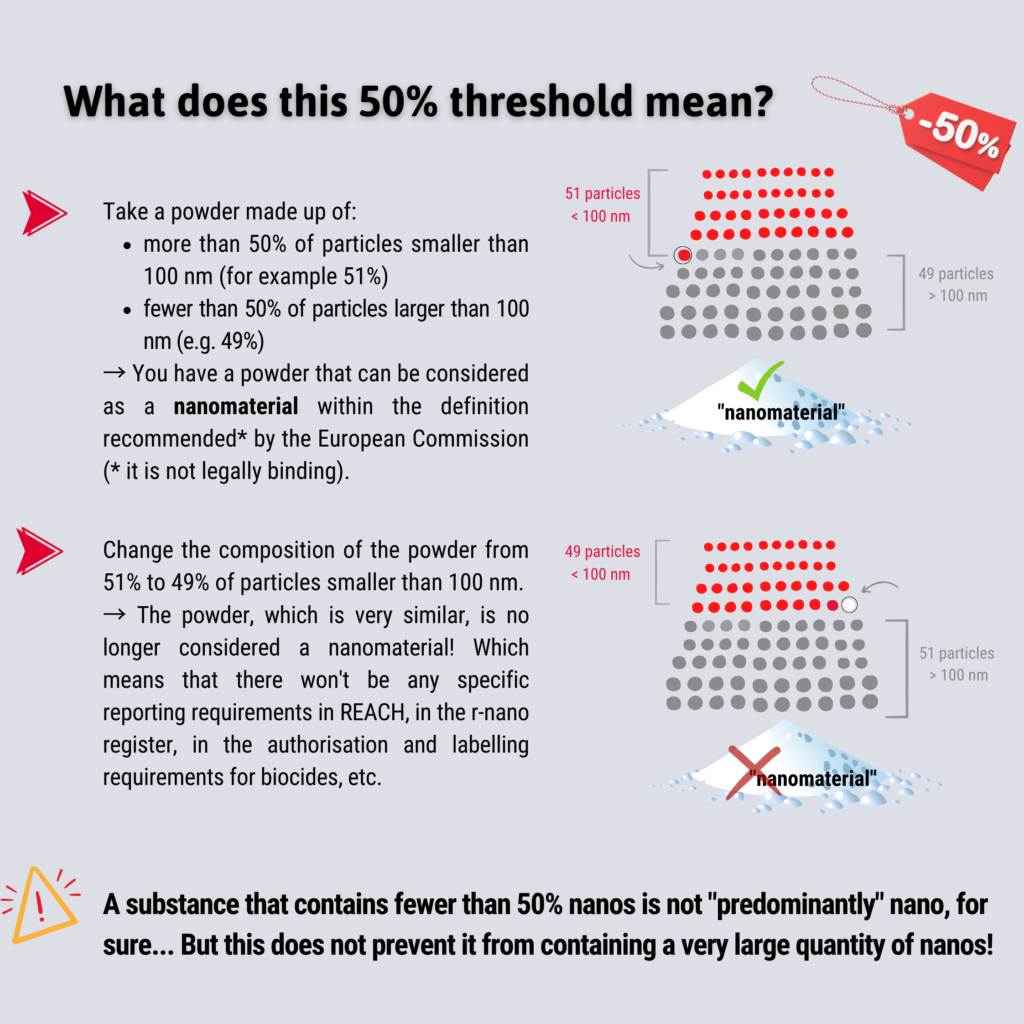
Removal of the possibility of lowering the 50% threshold
The 50% rate of particles below 100 nm in number is identical to that of 2011 (and more than 333 times higher than the one defended by Scenihr (0.15%) in 201013 Cf. Scientific Basis for the Definition of the Term “nanomaterial”, SCENIHR, European Commission, 2010: Scenihr insisted on the knowledge of the size distribution of a nanomaterial and on the necessity of a distribution in number (more relevant than a distribution in mass defended by the industrialists). As a reminder, at the time, even the German industry did not ask for so much: it had argued for a rate of about 10% “only”).
However, the Commission had foreseen in 2011 that in case of concerns in terms of environmental or health risks this rate could be lowered below 50%. This is what the French authorities in particular have maintained14Cf.Nanomaterials – Evaluation of the national R-Nano declaration system, December 2020. The French High Council for Public Health (HCSP), in a 2018 report15Cf. Protéger les travailleurs et les personnes au voisinage de sites de production ou de manipulation de nanoparticules de dioxyde de titane, Haut conseil de la santé publique, 25 juin 2018, also recommended a 10% threshold by number for all substances and 1% by number in specific cases. ANSES16See https://www.anses.fr/en/content/seeking-more-protective-european-definition-nanomaterials also urges not to exclude from this definition certain nanomaterials that could be problematic for human health and the environment. According to the Agency, the definition must be as inclusive as possible. ANSES will publish an opinion, supported by a group of multidisciplinary experts, that will enrich the response to the European consultation (initially scheduled for 2022, the opinion is still not published in May 2023).
Industry federations, on the other hand, have opposed it17Cf. EU Plans to Review the Definition of a Nanomaterial, Nanotechnology Industries Association (NIA), December 3, 2020 and won their case during the revision of the recommendation, since the European Commission has removed this clause from the definition of 2022, which sets the rate at 50% without the possibility of lowering it.
This deletion now makes it possible not to consider as nanomaterials (and therefore not to regulate accordingly) substances of which 49% of the particles in number would be made up of particles below the 100 nm mark. How about health and environmental considerations?
Let’s take the particular case of cosmetics: the definition of the current Cosmetics Regulation does not include a threshold, the French authorities have used a threshold of 10% until now. Using a fixed threshold of 50%, would exempt from labeling and notification thousands of cosmetics containing nanoparticles, some of which are potentially dangerous for consumers.
What is the scope of this revised definition?
Towards transpositions with or without adaptation(s) in European regulations?
Will the revised definition be transposed to all Community regulations, regardless of their field of application? Not in the immediate future anyway: changing the definition of European regulations to bring them into line with the Commission’s definitional recommendation requires a specific co-decision review process that can take, in some cases, several years.
The Commission has clearly indicated its wish to see its revised recommendation become “binding”, from a legal point of view; it should therefore eventually be transposed into the regulation REACH (expected by the end of 2022) and other EU regulations with provisions on nanomaterials – starting with the cosmetics (work is in progress), then food, biocides and medical devices mainly (but what about the regulation on plant protection products as well as the draft revision of the directive on industrial emissions (IED) aimed at reducing emissions of polluting substances?)
Will this definition be transposed as is or with adaptations or management measures to minimize the health or environmental risks of materials “exhibiting nanoscale properties” but not covered by the revised definition recommendation?
The current revision of the Cosmetics Regulation18Cf. Towards a new nano definition in the future Cosmetics Regulation… yes, but which one?, VeilleNanos, July 13, 2022 were supposed to be, with REACH, the first “test” and could set a precedent. But time is flying and it seems that a delegated act aiming at copying the new definition into the Novel Foods regulation could come sooner than expected…
Health safety must prevail over legal consistency and certainty
The harmonization of the terminology was requested by the industry in order to put an end to the difficulties caused by the coexistence of different definitions which complicates the work of producers / importers / distributors: the latter complain about having to declare and label “nanomaterials” defined differently in various national registries (as r-nano in France), in REACH at European level and in sectoral regulations (food, cosmetics, biocides, medical devices, etc.). However, the harmonization of definitions must not be at the expense of public health and the environment. The problems associated with the coexistence of different definitions is a problem that could be solved upstream in the supply chain: if companies that produce and/or mix nanomaterials perform accurate and complete characterization of the substances and transmit the information correctly, downstream stakeholders can apply the appropriate definition to their sector and implement the appropriate labeling, registration and notification where applicable.
An operational and unique definition: “mission impossible”?
Defining what a nanomaterial is and revising the current definitions are complex problems, scientific but also highly political, with multiple implications for companies, lawyers and public authorities alike. The exercise is so perilous19For a synthetic analysis of the ins and outs of the debates around a single definition of nanomaterials, see the article by Georgia Miller and Fern Wickson “Disagreement Over Definitions Including Relevant Size, Size Distribution, Intentionality of Production, and Occurrence of Novel Properties” in Risk Analysis of Nanomaterials: Exposing Nanotechnology’s Naked EmperorMiller G and Wickson F, Review of Policy Research, 32(4): 485, July 2015. that some scientists even advise against it. For a long time, the French authorities also did not push for the revision of the definition at the European level: it is indeed on the previous one that was built the R-Nano device of annual declaration of the “substances in the nanoparticulate state”, obligatory in France since 2013.
NGOs are also sceptical20At the March 2014 CASG nano meeting, the European Environmental Bureau (BEE) criticized the Commission’s eagerness to launch this project: advances in the characterization and metrology of nanomaterials have certainly evolved since 2011, but not enough, according to the EEB, to justify a revision of the definition. This project, which is necessarily time-consuming and involves significant public expenditure, is less important and urgent than others, which were neglected by the Commission at the time, notably :
– the adaptation of the REACH annexes to nanomaterials (finally adopted in 2018, even if its implementation is still unfinished in mid-2020)
– the establishment of a Community register of nanoproducts on the European market..
Like Andrew Maynard before them21Don’t define nanomaterials, Maynard A, Nature, 475, July 2011, French researchers consider that “an institutional definition is not a sine qua non condition for risk management and that the absence of definition or, in this case, the multiplicity of definitions from multiple sources, does not create a vacuum or exacerbate the difficulties of understanding risk management issues“. The researchers also consider that “a single definition, if it wants to remain operational, cannot embrace all the complexity of the questions related to the nano state of a particle. A scientific definition of nanoparticles and nanomaterials is not specifically useful to study whether or not they present a risk to health or more broadly to the environment“22CERTOP, The mobility of “nanos” risks, September 2014.
What are the strategies of the companies that produce and/or use nanos?
Industrialists may resort to avoidance strategies like the development of nanomaterials whose size and distribution in number can flirt with the thresholds (with a little more than 50% of the particles exceeding 110 nm for example) in order to escape the regulations while retaining the desired properties.
While manufacturers may have an interest in advocating for the narrowest possible definition of nanomaterials, in order to remove as many materials as possible from the regulations, market leaders may have an interest in ensuring that the definition and disclosures (particularly in the context of REACH) be very demanding, which would give them a competitive advantage over other companies with lesser means (technical and financial).
Another way to proceed, more in line with Corporate Social Responsibility (CSR), is to take part in a co-vigilance approach: anticipate the impacts of the massive use of these materials with specific properties of the nanometric scale and their release into the environment in order to give priority to eco-design of less dangerous materials (“safe by design”) and to minimize the risks throughout the life-cycle, sharing information with other actors upstream and downstream of the production chain (up to the final consumer), etc.
To be continued…
In French:
- Legal controversy over the definition of nanomaterials in European law,
- The Commission publishes its new definition of nanomaterials, Actu Environnement, 21 July 2022
- The European recommendation for the definition of a nanomaterial is evolving, LNE, June 15, 2022
- Scientific and technical support note on “the development of a proposal for an updated definition of the term “nanomaterials” based on Recommendation 2011/696/EU”, Anses, 14 January 2022
- Definition of nanomaterials: a small step towards a revision?Actu Environnement, September 10, 2021
- Nanomaterials: for a more protective European definition, Anses, 23 July 2021
- Review of available analytical methods for the characterization of nano-objects, their aggregates and agglomerates in order to meet regulatory requirements, Anses, February 2020
- Nanoparticles: towards a consolidated European definition by the end of the year?, Actu Environnement, July 2017
In English:
- European nanomaterial legislation in the past 20 years – Closing the final gaps, Nielsen MB et al., NanoImpact, 2023
- ANSES calls for the adoption of a more protective definition for nanomaterials, Anses, May 2023
- Definition of a nanomaterial, European Commission
- Substance in nanomaterials regulation, Quinn BM, Nature Nanotechnology, 16: 1172-1175, 2021
- EU Plans to Review the Definition of a Nanomaterial, Nanotechnology Industries Association (NIA), December 3, 2020
- “An overview of concepts and terms used in the European Commission’s definition of nanomaterial,” Joint Research Center (JRC), February 2019
- Nanomaterials or non-nanomaterials, JRC, March 30, 2017
- Reliable nanomaterial classification of powders using the volume-specific surface area method, Journal of Nanoparticle Research, 19:61, February 2017
- Nanomaterials Definition fact sheet, Öko-Institut, CIEL and ECOS, November 2014
Any questions or comments? This information sheet compiled by AVICENN is intended to be completed and updated. Please feel free to contribute.
More news on the topic
Upcoming Nano Agenda

- Webconference for analysis laboratories, plant fertilizer manufacturers and distributors, public authorities…
- Moderated by David Krupka, nanotechnologies development manager at AFNOR Normalisation and Emilie Langlois-Bertrand, nantechnologies standardization project manager.
- In partnership with Armand Masion (CEREGE) and Patrice Charpentier (ANSES).
- This exchange will also be an opportunity to explore the creation of a national platform to identify standardization needs.
- Website: https://www.afnor.org/evenements/qualite/nanotechnologies-agriculture-cadre-pratique-responsable

- International conference on metallic nano-objects for experts working in the interdisciplinary field of metallic nanoparticles, with a particular emphasis on nanoparticle synthesis and characterization, plasmonics, optics and photonics, catalysis, biomedicine, electronics, and nanoparticle recycling
- Organizers / Partners: CNRS, Bordeaux University, Bordeaux INP, ICMCB, CRPP, CBMN, ISM
- Website: https://mno2026.sciencesconf.org
- Two days of information, discoveries and testimonials for occupational health and safety professionals, experts and enthusiasts to discuss chemical risk prevention in the workplace.
- Organizers: Association TOXILIST
- Website: https://toxidays.fr/
Sheet originally created on April 9, 2014
Notes and references
- 1In 2014 and 2015 the JRC published an initial analysis report (See: Towards a review of the EC Recommendation for a definition of the term “nanomaterial” – Part 1: Compilation of information concerning the experience with the definition, JRC, March 2014), followed by an evaluation report (Towards a review of the EC Recommendation for a definition of the term “nanomaterial” – Part 2: Assessment of collected information concerning the experience with the definitionJRC, September 2014) and then a third report of recommendations to improve the content and implementation of the future definition (See Towards a review of the EC Recommendation for a definition of the term “nanomaterial”: Part 3: Scientific-technical evaluation of options to clarify the definition and to facilitate its implementation, JRC, July 2015)
- 2In early May 2017, there were rumblings of an imminent release… and in early July, the trade press was talking about a summer release. In particular, the Commission confirmed, in mid-March 2017, before the Casg nano, that its draft revision would be subject to a 6-week public consultation after its publication, in order to be adopted in the second half of 2017 and accompanied by an explanatory note produced by the Joint Research Centre (JRC) attached to the Commission. A public and stakeholder consultation on the “roadmap ” did take place from mid-September to mid-October 2017, but it focused on the methodology, goals, and general principles guiding the development of the revised definition, more so than on the specific content of the new definition. 18 contributions were made, by industrialists, environmental agencies and some anonymous citizens. The roadmap mentions a 12-week consultation in the first quarter of 2018, but by the end of February 2019, feedback from Brussels indicated a more than likely postponement of the publication of the draft and the consultation to 2020 (see EU revision of nanomaterials definition postponed to 2020, Chemical watch, February 26, 2019)
- 3Initially published on the following link https://ec.europa.eu/environment/pdf/chemicals/nanotech/TSC_Nanodefinition_PublicExcerpt.xlsx, they seem to have disappeared from the European Commission’s website; AVICENN had downloaded them in 2021 and has decided to upload them to veillenanos.fr in order to keep track of the positions defended at that stage by the stakeholders
- 4despite the details provided by the Commission staff working document (SWD(2022) 150)
- 5Cf. Scientific and technical support note on “the development of a proposal for an updated definition of the term “nanomaterials” based on Recommendation 2011/696/EUAnses, January 14, 2022; Opinion and report on the request for scientific and technical support in drafting a proposal for an updated definition of the term “nanomaterial” based on European Commission Recommendation 2011/696/EU on the definition of nanomaterial, Anses, May 2023
- 6Cf. La Commission publie sa nouvelle définition des nanomatériaux, Actu Environnement, 21 juillet 2022
- 7Controverses juridiques sur la définition des nanomatériaux en droit européen, Marion Tissier-Raffin, Nickolas Achat, Didier Morin, Journal d’actualité du droit international et européen (JADIE), n°24, novembre 2024
- 8See in particular:
– Nanomaterials: for a more protective European definition, Anses, 23 July 2021
– Contribution of AVICENN to the European consultation on the definition of the term “nanomaterial”, AVICENN, June 30th 2021
– Scientific Basis for the Definition of the Term “nanomaterial”, SCENIHR, European Commission, 2010 - 9Cf. Reporting Format for Nanotechnology-Related Information in CMC Review, Office of Pharmaceutical Science (FDA), June 2010
- 10Bai Y et al, 100 Nanometers: A Potentially Inappropriate Threshold for Environmental and Ecological Effects of Nanoparticles, About. Sci. Technol., 48 (6) : 3098-3099, 2014
- 11The International Organization for Standardization (ISO) defines a nanomaterial as “a material with any external dimension at the nanoscale or internal or surface structure at the nanoscale” (the nanoscale is defined as a spectrum of dimensions from about 1 nm to 100 nm); it has adopted the term NOAA to encompass all “Nano-Objects, their Agglomerates and Aggregates greater than 100 nm”
- 12
- 13Cf. Scientific Basis for the Definition of the Term “nanomaterial”, SCENIHR, European Commission, 2010: Scenihr insisted on the knowledge of the size distribution of a nanomaterial and on the necessity of a distribution in number (more relevant than a distribution in mass defended by the industrialists)
- 14Cf.Nanomaterials – Evaluation of the national R-Nano declaration system, December 2020
- 15Cf. Protéger les travailleurs et les personnes au voisinage de sites de production ou de manipulation de nanoparticules de dioxyde de titane, Haut conseil de la santé publique, 25 juin 2018
- 16See https://www.anses.fr/en/content/seeking-more-protective-european-definition-nanomaterials
- 17Cf. EU Plans to Review the Definition of a Nanomaterial, Nanotechnology Industries Association (NIA), December 3, 2020
- 18Cf. Towards a new nano definition in the future Cosmetics Regulation… yes, but which one?, VeilleNanos, July 13, 2022
- 19For a synthetic analysis of the ins and outs of the debates around a single definition of nanomaterials, see the article by Georgia Miller and Fern Wickson “Disagreement Over Definitions Including Relevant Size, Size Distribution, Intentionality of Production, and Occurrence of Novel Properties” in Risk Analysis of Nanomaterials: Exposing Nanotechnology’s Naked EmperorMiller G and Wickson F, Review of Policy Research, 32(4): 485, July 2015.
- 20At the March 2014 CASG nano meeting, the European Environmental Bureau (BEE) criticized the Commission’s eagerness to launch this project: advances in the characterization and metrology of nanomaterials have certainly evolved since 2011, but not enough, according to the EEB, to justify a revision of the definition. This project, which is necessarily time-consuming and involves significant public expenditure, is less important and urgent than others, which were neglected by the Commission at the time, notably :
– the adaptation of the REACH annexes to nanomaterials (finally adopted in 2018, even if its implementation is still unfinished in mid-2020)
– the establishment of a Community register of nanoproducts on the European market. - 21Don’t define nanomaterials, Maynard A, Nature, 475, July 2011
- 22CERTOP, The mobility of “nanos” risks, September 2014



