
Nanos in water: Release, fate and effects on fauna and flora

By the AVICENN team – Last updated August 2022
Release, fate and effects on fauna and flora of nanomaterials in water
Release of nanomaterials in water
What quantity of nanomaterials is released in water?
The quantity of nanoparticles released in water is unknown today. One of the major challenges is that nanoparticles are not well detected in water at low concentrations.
Different models1See in particular
– Nano silver and nano zinc-oxide in surface waters – Exposure estimation for Europe at high spatial and temporal resolution, Dumont E et al, Environmental Pollution, 196: 341-349, January 2015
– Gottschalk F et al, Probabilistic material flow modeling for assessing the environmental exposure to compounds: methodology and an application to engineered nano-TiO2 particles, Environ Model Software, 25:320-32, 2010
– Blaser SA et al, Estimation of cumulative aquatic exposure and risk due to silver: contribution of nano-functionalized plastics and textiles, Sci Total Environ, 390:396-4092008, February 2008
– Boxall ABA et al, Current and predicted environmental exposure to engineered nanoparticles, York: CSL, 2008
– Mueller NC & Nowack B, Exposure modelling of engineered nanoparticles in the environment, Environ Sci Technol, 42:4447-53, 2008 have been tried in an attempt to quantify the concentrations and fluxes of different types of manufactured nanoparticles in the environment. However, these exercises are based primarily on estimates of the quantities of manufactured nanomaterials produced rather than estimates of the quantities of manufactured nanomaterials contained in consumer products2Cf. How important is drinking water exposure for the risks of engineered nanoparticles to consumers, Tiede K et al, Nanotoxicology, 1-9, 2015.
In 2013, researchers estimated that between 0.4 and 7% of the 300,000 tons of engineered nanomaterials produced worldwide in 2010 were released in water3Cf. Global life cycle releases of engineered nanomaterials, Keller AA et al, Journal of Nanoparticle Research, 15:1692, May 2013. But these figures are far below the reality since we learned the same year thanks to the French mandatory declaration that no less than 500,000 tons of “substances in a nanoparticulate state” had been produced or imported on French territory alone in 2013.
How do nanomaterials get released in water?
Nanomaterials can be released in water:
- in industrial effluents from companies where nanomaterials are produced / handled / processed
- when swimming after having applied sunscreen containing nanoparticles4In 2014, Spanish researchers estimated that tourist activity on a Mediterranean beach during a summer day can release about 4 kg of titanium dioxide nanoparticles in the water, resulting in an increase of 270 nM/day in the concentration of hydrogen peroxide (a molecule with toxic potential, particularly for phytoplankton, which is the basic food of marine animals). Cf. Nanos UV screens: a danger for marine life, L’Observatoire des Cosmétiques, September 5, 2014
Researchers from CEREGE in France have measured the concentration of titanium in the water of three beaches in Marseille and have estimated the weight of TiO2 released in two months of summer for a small beach at 54 kilos per day. See :
– Doc’ en clip – the risk associated with nanoparticles in sunscreens (video), Riccardo Catalano, Aix-Marseille University, October 14, 2019
– Scientists find titanium dioxide from sunscreen is polluting beaches Scientists find titanium dioxide from sunscreen is polluting beaches, presentation by Labille J., Goldschmidt Conference, August 2018
– Estimation and minimization of the risk associated with TiO2 nanoparticles used in sunscreens, presentation by Labille J, “Nano and cosmetics” technical day organized by LNE, 29 March 2018
– Pollution of coastal waters by UV absorbers from sunscreens, generated by summer activities, Labille J, OHM Littoral project, 2017 - when washing textiles on which nanoparticles have been applied5See in particular:
– Silver nanoparticles lost in the first wash, Chemistry World, 30 March 2016 and Durability of nano-enhanced textiles through the life cycle: releases from landfilling after washing, DM Mitrano et al, Environ. Sci.: Nano, 2016
– Presence of Nanoparticles in Wash Water from Conventional Silver and Nano-Silver Textiles, Mitrano DM et al, ACS Nano, 2014 - from rainwater runoff from the cements and exterior paints covered with nanocoatings6Titanium dioxide nanoparticles have been detected in water runoff from walls painted with paints containing this substance: Synthetic TiO2 nanoparticle emission from exterior facades into the aquatic environment, Kaegi R. et al, Environmental Pollution, 156(2), 2008 and following the leaching of contaminated soils
- from corroding paints applied to boats (some contain copper oxide nanoparticles to prevent small crustaceans and mussels from attaching to the hull7Nanomaterials in sunscreens and boats leave marine life vulnerable, UC Davis News, May 12, 2015 (press release))
- by the deposition of atmospherically transported particles
- following an accidental spill.
Which nanomaterials are most likely to be present in water?
Because of the uncertainties about the volumes of nanomaterials marketed and released in water, scientists’ estimates are not consistent and vary according to the methods and assumptions used and the practices of different countries (e.g. land application of sewage sludge versus incineration):
- According to a recent study, the nanoparticles with the highest potential concentrations in treated water in the UK are titanium dioxide nanoparticles and zinc nanoparticles (from sunscreens and other cosmetics) and silica nanoparticles (toothpaste); carbon, iron or silver nanoparticles come only in 6th, 7th and 8th position respectively, and cerium oxide has the weakest concentration8Cf. How important is drinking water exposure for the risks of engineered nanoparticles to consumers, Tiede K et al, Nanotoxicology, 1-9, 2015.
- According to another estimate, this time for Denmark, the highest concentrations of nanoparticles in aquatic systems are for carbon black and Photostable TiO2 (contained in sunscreens and not in photocatalytic paints), followed by copper carbonate (CuCO3, assuming its use as a wood preservative will increase). Water treatments would lead to extremely low concentrations of zinc oxide (ZnO) and silver nanoparticles in the environment9Modeling Flows and Concentrations of Nine Engineered Nanomaterials in the Danish Environment, Gottschalk F et al., Int. J. About. Res. Public Health, 12(5), 5581-5602, 2015.
In France, researchers have noted an increase in the presence of silver in the Gironde estuary10Silver (Ag, nanoAg) as an emerging contaminant in the Gironde estuary: scientific assessments and risk governance, Salles D. et al, ERS, 12: 317-323, July/August 2013 whose causes since 2005 are still poorly known, but potentially linked:
- to agricultural soil erosion
- to cloud seeding (silver iodide solution) to avoid hail impacts on vine crops and arboriculture
- to community wastewater discharges.
Fate of nanomaterials in the aquatic environment
In water, nanos can undergo modifications
Knowledge about what happens to nanomaterials in water is beginning to develop but is still very limited. Because of their small size and especially their high reactivity, nanomaterials tend to interact with almost all the elements present in water (mineral, chemical or biological materials), according to very variable configurations depending on their physicochemical characteristics and the composition of the medium.
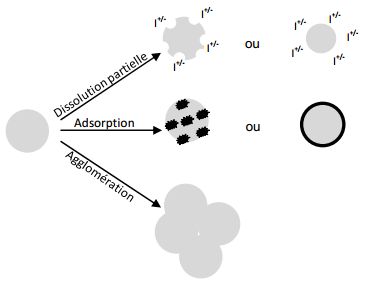
Thus, in water, nanomaterials can undergo the following modifications:
- phenomena of dissolution can intervene (more particularly on silver and zinc oxide nanoparticles) and be all the more important as the particles are small12Aggregation and Dissolution of 4 nm ZnO Nanoparticles in Aqueous Environments: Influence of pH, Ionic Strength, Size, and Adsorption of Humic Acid, Bian SW et al, Langmuir, 27 (10), pp 6059-6068, 2011
- phenomena of adsorption can lead to the fixation of other elements (including pollutants) on the surface of nanoparticles
- phenomena of agglomeration of nanomaterials have been observed in natural waters13Aggregation and Dissolution of 4 nm ZnO Nanoparticles in Aqueous Environments: Influence of pH, Ionic Strength, Size, and Adsorption of Humic Acid, Bian SW et al, Langmuir, 27 (10), pp 6059-6068, 2011.
Some nanomaterials tend to sediment by gravity and accumulate in sediments (especially in the case of aggregated and/or hydrophobic nanomaterials such as carbon nanotubes14A research team in the USA has shown the accumulation of single-walled carbon nanotubes in wetland sediments: Fate of single walled carbon nanotubes in wetland ecosystems, Schierz A et al, Environ. Sci: Nano, 2014 (see also associated press release: Nanoparticles accumulate quickly in wetlands, Science Daily, October 1, 2014)) which increases the risk of contact with microorganisms living on aquatic sediments. On the contrary, others would tend to remain in suspension (especially if coated with a hydrophilic surface coating) and disperse easily, increasing the risk of exposure15See in particular Transport of nanoparticulate TiO2 UV-filters through a saturated sand column at environmentally relevant concentrations, Motellier D et al, Science of the Total Environment, 811, 152408, March 2022 and the references cited in the report Toxicity and ecotoxicity of carbon nanotubes, ANSES, February 2011.
Their degradation, or conversely their persistence, are also complex to determine and vary according to the nanomaterials and the quality of the water:
- According to a 2011 study, carbon nanomaterials (C60 fullerenes, carbon nanotubes) are not biodegradable in liquid media in the environmentBiodegradability16of organic nanoparticles in the aqueous environment, Kummerer K et al, Chemosphere, 82(10):1387-92, 2011.
- Another study, the results of which were also made public in 201117Cf. Les nanoparticules : quels risques en Seine ?, Yann Sivry et al., communication aux 22èmes Journées Scientifiques de l’Environnement – Reconquête des environnement urbains : les défis du 21ème siècle, février 2011 was conducted on nanoparticles of zinc oxides (ZnO) and titanium dioxide (TiO2) in Seine river water, and showed that:
- the nanoparticulate form of TiO2 is not more soluble than its microparticulate or macroparticulate counterparts
- on the other hand, a large proportion of zinc oxide nanoparticles are rapidly dissolved in Seine water
- the coating, depending on its nature, can decrease or increase the dissolution of nanoparticles.
Nanomaterials in general would tend to be stabilized in media with low ionic strength and high dissolved organic carbon content (COD)18Cf. A review of the properties and processes determining the fate of engineered nanomaterials in the aquatic environment, Peijnenburg W et al, Critical Reviews in Environmental Science and Technology, 45(19): 2084-2134, 2015.
Nanomaterials are already found in wastewater treatment plants and industrial water treatment plants but the treatments in place have not been designed to filter them. A significant part ends up in surface waters, while the others accumulate in the sludge of wastewater treatment plants spread on agricultural land…
In the USA, Marie Simonin has conducted impact studies of NP in mesocosms with CIENT. In 2018 she co-authored two publications in English:
– one shows that the biotransformation of nanoparticles should not be ignored, even for nanoparticles generally considered stable in the environment (here gold nanoparticles).
– the other is studying the impacts of a citrate-coated gold nanoparticle and a commercial pesticide containing copper (OH) nanoparticles on aquatic primary producers under ambient and enriched nutrient conditions (this mimics fertilizer releases). Wetland mesocosms were repeatedly exposed with low concentrations of nanoparticles and nutrients during a nine-month experiment to replicate realistic field exposure scenarios. In the absence of nutrient enrichment, there were no persistent effects of gold or copper nanoparticles on primary producers or ecosystem productivity. However, combined with nutrient enrichment, both types of nanoparticles intensify their eutrophication. When either nanoparticle was added in combination with nutrients, the algal blooms persisted 50 days longer than in the nutrient-only treatment. These two emerging contaminants and synthetic chemicals may play an underappreciated role in global trends of increasing eutrophication. The study shows that chronic exposure to gold and copper oxide nanoparticles at low concentrations can intensify the eutrophication of wetlands and promote algal blooms.
Transfer through porous environments
Transfer processes through a porous medium (soil or aquifer) are also the subject of research. Experiments are performed in the presence of a solid phase or through a solid phase. They allow a better understanding of the adsorption processes, the impact of hydrodynamics and aggregation on the transport processes of nanoparticles. However, the first experiments were carried out on very simplified models such as silica beads. Trials in more complex environments with multiple minerals and natural organic matter are only beginning to develop19Background note – “Pollution” workshop – “Reducing pollution and impacts on biodiversity” – April 2010 (for the French Biodiversity Conference in May 2010).
What effects do nanomaterials have on aquatic fauna and flora?
The contamination of water by manufactured nanoparticles or their residues also leads to the contamination of aquatic organisms such as algae, shellfish and fish. Studies on the effects of nanomaterials on aquatic fauna and, to a lesser extent, on aquatic flora are developing, but many uncertainties remain (the salinity or acidity of the water can modify their toxicity, for example) and concerns are high.
The transfer of nanomaterials in the food chain
It is already known that nanomaterials or nanomaterial residues can enter and accumulate in different aquatic species, be transferred from generation to generation and move up the food chain.
Researchers have demonstrated the transfer of nanomaterials from seawater to the digestive tract of mussels20Uptake and retention of metallic nanoparticles in the Mediterranean mussel (Mytilus galloprovincialis), Aquatic Toxicology, May 2013, from algae to zooplankton and then to the fish that feed on them21See for example Evidence for Biomagnification of Gold Nanoparticles within a Terrestrial Food Chain, Judy. J et al., About. Sci. Technol., 45 (2), 776-781 (2011); Food Chain Transport of Nanoparticles Affects Behaviour and Fat Metabolism in Fish, Cedervall T. et al, PLoS ONE, 7(2): e32254 (2012).
We speak of “biomagnification”: there is an increase in the toxic content from one link in the food chain to the next:
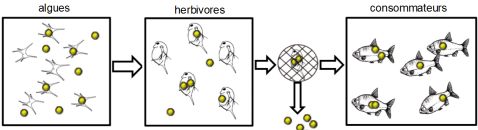
Even when altered and agglomerated, nanoparticles (including cerium dioxide, used as a protective anti-scratch UV agent in exterior paints) may retain their ecotoxicity to aquatic organisms (microalgae in the experiment conducted)22Prise en compte de l’évolution de l’état d’agglomération dans l’étude de l’écotoxicité des nanoparticules, Nicolas Manier, Rapport scientifique 2013-2014, INERIS, November 2014, p.16.
Some examples of effects observed
Some examples of effects since 201123See for example:
– Comparative evaluation on the toxic effect of silver (Ag) and zinc oxide (ZnO) nanoparticles on different trophic levels in aquatic ecosystems: A review, Sibiya A et al, Journal of applied toxicology, 2022 Nanomaterials interact with agricultural pesticides, increasing toxicity to fish, The Organic Center, February 2015 (lay summary of scientific article Ecotoxicological effects of carbofuran and oxidised multiwalled carbon nanotubes on the freshwater fish Nile tilapia: Nanotubes enhance pesticide ecotoxicity, Ecotoxicology and Environmental Safety, 111: 131-137, January 2015)
– Fate of single walled carbon nanotubes in wetland ecosystems, Schierz A et al, Environ. Sci.: Nano, 2014 (and associated press release: Nanoparticles accumulate quickly in wetlands: Aquatic food chains might be harmed by molecules ‘piggybacking’ on carbon nanoparticles, Science Daily, October 1, 2014
– Spatial distribution, electron microscopy analysis of titanium and its correlation to heavy metals: Occurrence and sources of titanium nanomaterials in surface sediments from Xiamen Bay, China, Luo Z et al, J. About. Monit., 13, 1046-1052, 2011: this study of sediments from Xiamen Bay in China showed that these sediments contained up to 2.74 g Ti/kg, largely in the form of 300 nm agglomerates composed of nanoparticles of about 50 nanometers. The distribution of titanium in sediments is positively correlated to that of elements such as lead or zinc, which is consistent with the adsorption of pollutants on the surface of nanoparticles.:
- effects on algae: increased mortality, growth retardation, decreased photosynthesis and generation of reactive oxygen species
- effects on crustaceans: increased mortality, behavioral changes, malformations in Daphnia, accumulation in the body
- effects on fish: mortality and disruption of development with the appearance of malformations; nano silver in particular can cause very marked malformations in the embryo of zebrafish
- effects on other aquatic organisms:
- damage throughout the mussel’s body, including induction of inflammatory processes, increased expression of genes involved in stress regulation, increased activity of antioxidant enzymes and lipid peroxidation
- toxic effects on freshwater snails, chironomid larvae, cnidarians and polychaetes: decreased nutrition, increased malformations, oxidative stress, DNA damage correlated with increased mortality
- toxic effects on amphibians
- At high concentrations, effects of carbon nanotubes have been observed on aquatic organisms: decreased fertilization rate in small crustaceans, malformations, delayed hatching and even increased mortality rate of zebrafish embryos.
In addition to these effects, there are worrying indirect damages
In addition to the toxic effects that they can induce directly, nanomaterials can cause indirect damage that is nonetheless of great concern:
- Nanomaterials or their residues can pass through the cell wall of plants or animals and bring in external molecules (this is the “Trojan horse” effect), they can act as “vectors” and promote the transport of pollutants (heavy metals, PAHs or pesticides for example).
- Nanomaterials can weaken plants or animals:
- Researchers in the USA have just demonstrated that zinc and copper oxide nanomaterials, even at low concentrations, can make sea urchin embryos more sensitive to other contaminants24Cf. Nanomaterials in sunscreens and boats leave marine life vulnerable, UC Davis News, May 12, 2015 (press release); Copper oxide and zinc oxide nanomaterials act as inhibitors of multidrug resistance transport in sea urchin embryos: Their Role as Chemosensitizers, WU B et al., About. Sci. Technol., 49 (9): 5760-5770, April 2015.
- Other German and American researchers have recently highlighted the fact that nanoparticles of titanium dioxide can disrupt the immune system of fish (minnows) and their resistance to bacterial pathogens25Cf. Titanium dioxide nanoparticles enhance mortality of fish exposed to bacterial pathogens, Jovanovic B et al, Environmental Pollution, 203: 153-164, August 2015. This makes it difficult for them to survive in the event of illness.
- Other studies are conducted with equally worrying conclusions26See in particular
– Ecotoxicological Effects of Transformed Silver and Titanium Dioxide Nanoparticles in the Effluent from a Lab-Scale Wastewater Treatment System, Georgantzopoulou A et al., About. Sci. Technol., 52, 16, 9431-9441, 2018
– The true effects of nanoparticles in their environment, CORDIS, March 2018 : “Most synthetic nanomaterials emitted into the environment will sooner or later arrive in our oceans and seas. The SOS-Nano project designed tests to predict their toxicity to the marine environment. The researchers used an ingenious natural in vivo water exposure system to test the effects of metal oxide nanoparticles: zinc oxide (ZnO) and manganese dioxide (MnO2). Oyster larvae suffered from a high level of toxicity occasioned by ZnO, in contrast, MnO2 NPs were not toxic in all exposure scenarios.”.
- Nanomaterials, combined with other substances, could become (even) more dangerous: This is called the “cocktail effect“. “Studies agree that the presence of nanoparticles in a liquid medium leads to a greater accumulation of pollutants in organisms. The risks for the food chain up to humans are therefore real, both because of the nanoparticles themselves as well as through their role as a vector of contamination”27What interactions between nanoparticles and other environmental contaminants, Camille Larue, Bulletin de veille scientifique (BVS), Anses, December 2014.
Release of nanomaterials in water:

- In French :
- Guide pratique des micropolluants dans les eaux du bassin Seine-Normandie, AESN and INERIS, 2008, revised in 2017. The book contains sheets for metallic nanoparticles: Nickel, chromium, copper, zinc, aluminum, silver, cobalt, titanium, selenium.
- Silver (Ag, nanoAg) as an emerging contaminant in the Gironde estuary: scientific assessments and risk governance, Salles D. et al, ERS, 12: 317-323, July/August 2013
- In English:
- Sewage spills are a major source of titanium dioxide engineered (nano)-particle release into the environment, Loosli F et al, Environ. Sci.: Nano, 6, 763-777, 2019
- Engineered nanomaterials from wastewater treatment & stormwater to rivers, Final conference of the COST Action ES1205, Aveiro (Portugal), 7-8 February 2017
- Modeling Flows and Concentrations of Nine Engineered Nanomaterials in the Danish Environment, Gottschalk F et al., Int. J. About. Res. Public Health, 12(5), 5581-5602, 2015
- How important is drinking water exposure for the risks of engineered nanoparticles to consumers, Tiede K et al, Nanotoxicology, 1-9, 2015
- Sources, Distribution, Environmental Fate, and Ecological Effects of Nanomaterials in Wastewater Streams, Kunhikrishnan A et al, Critical Reviews in Environmental Science and Technology, 45(4), January 2015
- Presence of Nanoparticles in Wash Water from Conventional Silver and Nano-silver Textiles, ACS Nano, 8 (7), 7208-7219, June 2014
- Validation and sensitivity of the FINE Bayesian network for forecasting aquatic exposure to nano-silver, Science of The Total Environment, 473-474, 685-691, March 2014
- Sequential Studies of Silver Released from Silver Nanoparticles in Aqueous Media Simulating Sweat, Laundry Detergent Solutions and Surface Water, About. Sci. Technol., 48 (13), pp 7314-7322, 2014
- Comprehensive modeling of environmental emissions of engineered nanomaterials, Sun TY et al, About. Pollut., 185, 69-76, 2014
- Environmental concentrations of engineered nanomaterials: Review of modeling and analytical studies, Gottschalk, F et al, , About. Pollut., 181, 287-300, 2013
- Particle Flow Analysis: Exploring Potential Use Phase Emissions of Titanium Dioxide Nanoparticles from Sunscreen, Paint, and Cement, Arvidsson R et al, Journal of Industrial Ecology, 16(3): 343-351, June 2012
- Assessing the Environmental Risks of Silver from Clothes in an Urban Area, Arvidsson R et al, Human and Ecological Risk Assessment, 20(4), June 2012
- The Behavior of Silver Nanotextiles during Washing, About. Sci. Technol., 43 (21), 8113-8118, 2009
– Fate and transformation of nanomaterials in the aquatic environment
- In French :
- What happens to gold nanoparticles in the environment? Labex Serenade, January 16, 2019
- Emerging pollutants: new challenges for groundwater management, SFH and BRGM, May 2016
- Study of the behavior of nanomaterials at the river-ocean boundary using large mesocosms, Pelletier E, UQAR (Université du Québec à Rimouski), intervention at the 83rd Acfas Congress, Colloquium 210 – Presence, persistence, fate and effects of nanomaterials in the environment, May 2015
- Silver nanoparticles in the natural environment: the case of an estuary, Millour M, UQAR (Université du Québec à Rimouski), intervention at the 83rd Congress of Acfas, Colloque 210 – Présence, persistance, devenir et effets des nanomatériaux dans l’environnement, May 2015
- Environmental implications of nanotechnology. State of the art on the fate of manufactured nanoparticles in surface water Labille J et al, Annual conference of the PIREN-Seine research program, February 2014
- “Fate of nanomaterials in the water ecosystem” in Impact de nanoparticules de TiO2 et de nanotubes de carbone sur les végétaux, thesis, Camille Larue, 2011
- Nanoparticles: what are the risks in the Seine? Yann Sivry et al, paper presented at the 22nd Journées Scientifiques de l’Environnement – Reconquering urban environments: the challenges of the 21st century, February 2011
- In English:
- Differential Reactivity of Copper- and Gold-Based Nanomaterials Controls Their Seasonal Biogeochemical Cycling and Fate in a Freshwater Wetland Mesocosm, Avellan A et al., About. Sci. Technol., 54, 3, 1533-1544, 2020
- Morphological transformation of silver nanoparticles from commercial products: modeling from product incorporation, weathering through use scenarios, and leaching into wastewater, Mohan S et al, Nanomaterials, 9(9), 1258, 2019
- Nanoparticle stability in lake water shaped by natural organic matter properties and presence of particulate matter, Slomberg DL et al, Science of the Total Environment, 656: 338-346, March 2019
- Gold nanoparticle biodissolution by a freshwater macrophyte and its associated microbiome, Avellan A et al, Nature Nanotechnology, (13): 1072-1077, 2018
- Gold nanoparticle biodissolution by a freshwater macrophyte and its associated microbiome, Simonin M et al, Nature nanotechnology, August 2018.
- Engineered nanoparticles interact with nutrients to intensify eutrophication in a wetland ecosystem experiment, article Marie Simonin et al, Ecological applications, June 2018.
- Emerging contaminants: fate, effects and environmental risks, Conference, The society of Environmental Toxicology and Chemistry (SETAC), May 2016
- Rethinking Stability of Silver Sulfide Nanoparticles (Ag2S-NPs) in the Aquatic Environment: Photoinduced Transformation of Ag2S-NPs in the Presence of Fe(III), Li L et al, About. Sci. Technol., 2016
- Modeling Flows and Concentrations of Nine Engineered Nanomaterials in the Danish Environment, Gottschalk F et al., Int. J. About. Res. Public Health, 12(5), 5581-5602, 2015
- Addressing the complexity of water chemistry in environmental fate modeling for engineered nanoparticles, Sani-Kast N. et al, Science of the Total Environment, 2015
- A review of the properties and processes determining the fate of engineered nanomaterials in the aquatic environment, Peijnenburg W et al, Critical Reviews in Environmental Science and Technology, 45(19): 2084-2134, 2015
- Sources, Distribution, Environmental Fate, and Ecological Effects of Nanomaterials in Wastewater Streams, Kunhikrishnan A et al, Critical Reviews in Environmental Science and Technology, 45(4), January 2015
- Nano silver and nano zinc-oxide in surface waters – Exposure estimation for Europe at high spatial and temporal resolution, Dumont E et al, Environmental Pollution, 196: 341-349, January 2015
- In Situ Chemical Transformations of Silver Nanoparticles along the Water-Sediment Continuum, About. Sci. Technol., 49 (1) : 318-325, 2015
- Heteroaggregation of titanium dioxide nanoparticles with model natural colloids under environmentally relevant conditions, Praetorius A. et al, Environmental Science and Technology, 48: 10690-10698, 2014
- Fate of single walled carbon nanotubes in wetland ecosystems, Schierz A et al, Environ. Sci.: Nano, 2014
- Transfer, Transformation and Impacts of Ceria Nanomaterials in Aquatic Mesocosms Simulating a Pond Ecosystem, Tella M et al, Environ Sci Technol, 48: 9004-9013, 2014
- Miljøstyrelsen (Environmental Protection Agency of Denmark), Environmental fate and behaviour of nanomaterials, September 2014
- Emerging patterns for engineered nanomaterials in the environment: a review of fate and toxicity studies, Garner KL, Keller AA, Journal of Nanoparticle Research, July 2014
- Environmental fate and effects of manufactured CeO2 nanomaterials (nanoceria), 2014. This CIENT study deals with the effects of nanoparticles of cerium dioxide or nanoceruse, a fuel additive.
- Fate of nanoparticles in the aquatic environment. Removal of engineered nanomaterials from the water phase under environmental conditionsQuik JTK, thesis manuscript, Radboud University Nijmegen, The Netherlands, 2013
- Evaluating nanoparticle breakthrough during drinking water treatment, Environmental Health Perspectives, 121(10):1161-1166, July 2013
- Global life cycle releases of engineered nanomaterials, Keller AA et al, Journal of Nanoparticle Research, 15:1692, May 2013
- Long term Transformation and Fate of Manufactured Ag Nanoparticles in a Simulated Large Scale Freshwater Emergent Wetland, Lowry GV et al., About. Sci. Technol., 46 (13): 7027-7036, July 2012
- Silver behaviour along the salinity gradient of the Gironde Estuary, Environ Sci Pollut Res, Lanceleur L et al, 20: 1352-66, July 2012
Effects on fauna and flora:
In French :
- Silver nanoparticles are toxic to aquatic organisms, France Diplomatie, 26 October 2018
- Interaction and accumulation of nanoparticles in aquatic organisms, thesis, INERIS, 2018 (to 2021?)
- Nanomaterials across a salinity gradient: exposure and ecotoxicological effects during their life cycle Carole Bertrand, thesis, 2016, with the participation of Laure Giamberini, in connection with the project NanoSALT supported by the ANR to understand the fate of Ag and CeO2 nanoparticles from textiles and paints.
- Presence, persistence, fate and effects of nanomaterials in the environment, 83rd Acfas Congress, Colloquium 210 , May 2015
- What interactions between nanoparticles and other environmental contaminants, Camille Larue, Bulletin de veille scientifique (BVS), Anses, December 2014
- Prise en compte de l’évolution de l’état d’agglomération dans l’étude de l’écotoxicité des nanoparticules, Nicolas Manier, Rapport scientifique 2013-2014, INERIS, November 2014, p.16
- Nano UV screens: a danger for marine life, L’Observatoire des Cosmétiques, September 5, 2014: Spanish researchers have thus estimated that tourist activity on a Mediterranean beach during a summer day can release about 4 kg of titanium dioxide nanoparticles in the water, and result in an increase of 270 nM/day in the concentration of hydrogen peroxide (a molecule with toxic potential, particularly for phytoplankton, which is the basic food of marine animals) → Popularized summary in French of the following article: Sunscreens as a Source of Hydrogen Peroxide Production in Coastal Waters, Sánchez-Quiles D and Tovar-Sánchez A, About. Sci. Technol., 48 (16), 9037-9042, 2014
- Wrong way to silver, Eawag, February 2014 → French lay abstract of the article Linking toxicity and adaptive responses across the transcriptome, proteome, and phenotype of Chlamydomonas reinhardtii exposed to silver, PNAS, February 2014
- Impact of nanomaterials on water bacteria, algae, crustaceans, fish, other aquatic organisms, a simplified aquatic food chain in Impact de nanoparticules de TiO2 et de nanotubes de carbone sur les végétaux, thesis, Camille Larue, 2011
- Nanoparticles in the water ecosystem, Larue C and Carrière M, Bulletin de veille scientifique, n°14, ANSES, June 2011
In English:
- How Nanosilver Gets Into Our Freshwater, and What We Need To Do About It, Lauren Hayhusrt, Fisheries Research Biologist, IISD Experimental Lakes Area, April 16, 2020
- Silver and titanium nanomaterials present in wastewater have toxic effects on crustaceans and fish cells, Norwegian Institute for Water Research (NIVA), November 2019
- A sub-individual multilevel approach for an integrative assessment of CuO nanoparticle effects on Corbicula fluminea, Koehle-Divo V et al, Environmental Pollution, 254, Part A, November 2019
- Changes in protein expression in mussels Mytilus galloprovincialis dietarily exposed to PVP/PEI coated silver nanoparticles at different seasons, Duroudier N et al, Aquatic Toxicology, 210: 56-68, May 2019
- The Toxicity of Nanoparticles to Organisms in Freshwater, Lekamge S et al, Reviews of Environmental Contamination and Toxicology, 10 November 2018
- Waterborne exposure of adult zebrafish to silver nanoparticles and to ionic silver results in differential silver accumulation and effects at cellular and molecular levels, Lacave JM et al, Science of The Total Environment, 642: 1209-1220, November 2018
- Toxicity and trophic transfer of P25 TiO2 NPs from Dunaliella salina to Artemia salina: Effect of dietary and waterborne exposure, Bhuvaneshwari M et al, Environmental Research, 160: 39-46, January 2018
- Emerging contaminants: fate, effects and environmental risks, Conference, The society of Environmental Toxicology and Chemistry (SETAC), May 2016
- The influence of salinity on the fate and behavior of silver standardized nanomaterial and toxicity effects in the estuarine bivalve Scrobicularia plana, Bertrand, C et al. , Environ Toxicol Chem, 2016
- Smaller silver nanoparticles more likely to be absorbed by aquatic life, UCLA study finds, UCLA News, October 7, 2015
- Titanium dioxide nanoparticles enhance mortality of fish exposed to bacterial pathogens, Jovanovic B et al, Environmental Pollution, 203: 153-164, August 2015
- Adapting OECD Aquatic Toxicity Tests for Use with Manufactured Nanomaterials: Key Issues and Consensus Recommendations, Petersen EJ et al, About. Sci. Technol., 49 (16) : 9532-9547, 2015
- Nanomaterials in sunscreens and boats leave marine life vulnerable, UC Davis News, May 12, 2015 (press release); Copper oxide and zinc oxide nanomaterials act as inhibitors of multidrug resistance transport in sea urchin embryos: Their Role as Chemosensitizers, WU B et al., About. Sci. Technol., 49 (9): 5760-5770, April 2015
- Chronic toxicity of silver nanoparticles to Daphnia magna under different feeding conditions, Aquatic Toxicology, 161, April 2015
- Evaluation of environmental stress by comet assay on freshwater snail Lymnea luteola L. exposed to titanium dioxide nanoparticles, Daoud A, Toxicological & Environmental Chemistry, 2015
- Ecotoxicological effects of cerium dioxide nanoparticles in the aquatic environment: from an evaluation under monospecific conditions to the study of experimental food chains in microcosms, Agathe Bour, thesis, University of Toulouse, January 2015
- Sources, Distribution, Environmental Fate, and Ecological Effects of Nanomaterials in Wastewater Streams, Kunhikrishnan A et al, Critical Reviews in Environmental Science and Technology, 45(4), January 2015
- Silver nanoparticles could pose risk to aquatic ecosystems, European Commission DG Environment News Alert Service, issue 394, November 2014
- Toxicity of Physically and Chemically Made Silver Nanoparticles in Marsh Frog Tadpole (Rana ridibunda), International Journal of Environment and Sustainability, 3(3): 14-19, 2014
- Transfer, Transformation and Impacts of Ceria Nanomaterials in Aquatic Mesocosms Simulating a Pond Ecosystem, Tella M et al, Environ Sci Technol, 48: 9004-9013, 2014
- Sunscreens as a Source of Hydrogen Peroxide Production in Coastal Waters, Sánchez-Quiles D and Tovar-Sánchez A, About. Sci. Technol., 48 (16), 9037-9042, 2014
- Aquatic toxicity of manufactured nanomaterials: challenges and recommendations for future toxicity testing, Schultz A et al, Environmental Chemistry, 11(3) 207-226, 2014
- Toxicity of differently sized and coated silver nanoparticles to the bacterium Pseudomonas putida: risks for the aquatic environment? Matzke M, Jurkschat K, Backhaus T, Ecotoxicology, 23(5):818-29, July 2014 (see the abstract and commentary by Camille Larue in French in the Science Watch Bulletin of the ANSES of July 2014 here).
- Linking toxicity and adaptive responses across the transcriptome, proteome, and phenotype of Chlamydomonas reinhardtii exposed to silver, PNAS, February 2014
- Particle Size and Agglomeration Affect the Toxicity Levels of Silver Nanoparticle Types in Aquatic Environment, Ecopersia, 1 (3), 273-290, November 2013
- The toxicity of silver nanoparticles to zebrafish embryos increases through sewage treatment processes, Ecotoxicology, 22(8), 1264-1277, October 2013
- Ecotoxicological Aspects of Nanomaterials in the Aquatic Environment, Schirmer K et al, in Safety Aspects of Engineered Nanomaterials, edited by Wolfgang Luther and Axel Zweck, 2013
- Exposure of juvenile Danio rerio to aged TiO2 nanomaterial from sunscreen, Fouqueray M et al, Environmental Science and Pollution Research, 20(5): 3340-3350, May 2013
- Assessing the Environmental Risks of Silver from Clothes in an Urban Area, Arvidsson R et al, Human and Ecological Risk Assessment, 20(4), June 2012
- Toxicity of copper oxide nanoparticle suspensions to aquatic biota, Manusadianas L et al, About. Toxicol. Chem., 2;31:108-114, 2012
- Effects of aged TiO2 nanomaterial from sunscreen on Daphnia magna exposed by dietary route, Fouqueray M et al, Environmental Pollution, 163: 55-61, 2012
- Effects of metallic and metal oxide nanoparticles in aquatic and terrestrial food chains. Biomarkers responses in invertebrates and bacteria, Thiéry A et al, International Journal of Nanotechnology, 9(3-7), 181-203, 2012
Any questions or comments? This information sheet compiled by AVICENN is intended to be completed and updated. Please feel free to contribute.
Other news on the topic
Our information sheets to go further
Upcoming Nano Agenda

- International conference on metallic nano-objects for experts working in the interdisciplinary field of metallic nanoparticles, with a particular emphasis on nanoparticle synthesis and characterization, plasmonics, optics and photonics, catalysis, biomedicine, electronics, and nanoparticle recycling
- Organizers / Partners: CNRS, Bordeaux University, Bordeaux INP, ICMCB, CRPP, CBMN, ISM
- Website: https://mno2026.sciencesconf.org
- Toxicokinetics: the fate of chemicals in the body” training course:
- the different routes of entry for toxic products
- the importance of toxicokinetics in preventing substance toxicity,
- xenobiotic absorption, distribution, metabolism and elimination
- nanoparticle toxicity
- Organizer: Association Toxicologie Chimie (ATC)
- Date: March 26, 2026
- Speaker: Nicole Proust (Research Engineer, CNRS Honorary Research Director, Palaiseau)
- Website: www.atctoxicologie.fr/…

- E-learning program: a 2-hour awareness-raising session aimed at personnel potentially exposed to nanomaterials at the workplace, heads of laboratories or facilities where nanomaterials are handled, safety coordinators or engineers.
- Organizers: INSTN Grenoble (CEA)
- On the program:
- 1 – Introduction, definition and characteristics of nanomaterials
- 2 – Toxicity of nanomaterials: the state of knowledge
- 3 – Metrology and characterization of nanomaterials
- 4 – Prevention and protection against nanomaterials in the workplace
- 5 – Quiz: assessment of learning outcomes
- The 2-hour course can be viewed for one month from the date of registration.
- Website: https://instn.cea.fr/…risques-lies-aux-nanomateriaux…
Sheet originally created in October 2014
Notes and references
- 1See in particular
– Nano silver and nano zinc-oxide in surface waters – Exposure estimation for Europe at high spatial and temporal resolution, Dumont E et al, Environmental Pollution, 196: 341-349, January 2015
– Gottschalk F et al, Probabilistic material flow modeling for assessing the environmental exposure to compounds: methodology and an application to engineered nano-TiO2 particles, Environ Model Software, 25:320-32, 2010
– Blaser SA et al, Estimation of cumulative aquatic exposure and risk due to silver: contribution of nano-functionalized plastics and textiles, Sci Total Environ, 390:396-4092008, February 2008
– Boxall ABA et al, Current and predicted environmental exposure to engineered nanoparticles, York: CSL, 2008
– Mueller NC & Nowack B, Exposure modelling of engineered nanoparticles in the environment, Environ Sci Technol, 42:4447-53, 2008 - 2Cf. How important is drinking water exposure for the risks of engineered nanoparticles to consumers, Tiede K et al, Nanotoxicology, 1-9, 2015
- 3Cf. Global life cycle releases of engineered nanomaterials, Keller AA et al, Journal of Nanoparticle Research, 15:1692, May 2013
- 4In 2014, Spanish researchers estimated that tourist activity on a Mediterranean beach during a summer day can release about 4 kg of titanium dioxide nanoparticles in the water, resulting in an increase of 270 nM/day in the concentration of hydrogen peroxide (a molecule with toxic potential, particularly for phytoplankton, which is the basic food of marine animals). Cf. Nanos UV screens: a danger for marine life, L’Observatoire des Cosmétiques, September 5, 2014
Researchers from CEREGE in France have measured the concentration of titanium in the water of three beaches in Marseille and have estimated the weight of TiO2 released in two months of summer for a small beach at 54 kilos per day. See :
– Doc’ en clip – the risk associated with nanoparticles in sunscreens (video), Riccardo Catalano, Aix-Marseille University, October 14, 2019
– Scientists find titanium dioxide from sunscreen is polluting beaches Scientists find titanium dioxide from sunscreen is polluting beaches, presentation by Labille J., Goldschmidt Conference, August 2018
– Estimation and minimization of the risk associated with TiO2 nanoparticles used in sunscreens, presentation by Labille J, “Nano and cosmetics” technical day organized by LNE, 29 March 2018
– Pollution of coastal waters by UV absorbers from sunscreens, generated by summer activities, Labille J, OHM Littoral project, 2017 - 5See in particular:
– Silver nanoparticles lost in the first wash, Chemistry World, 30 March 2016 and Durability of nano-enhanced textiles through the life cycle: releases from landfilling after washing, DM Mitrano et al, Environ. Sci.: Nano, 2016
– Presence of Nanoparticles in Wash Water from Conventional Silver and Nano-Silver Textiles, Mitrano DM et al, ACS Nano, 2014 - 6Titanium dioxide nanoparticles have been detected in water runoff from walls painted with paints containing this substance: Synthetic TiO2 nanoparticle emission from exterior facades into the aquatic environment, Kaegi R. et al, Environmental Pollution, 156(2), 2008
- 7Nanomaterials in sunscreens and boats leave marine life vulnerable, UC Davis News, May 12, 2015 (press release)
- 8Cf. How important is drinking water exposure for the risks of engineered nanoparticles to consumers, Tiede K et al, Nanotoxicology, 1-9, 2015
- 9Modeling Flows and Concentrations of Nine Engineered Nanomaterials in the Danish Environment, Gottschalk F et al., Int. J. About. Res. Public Health, 12(5), 5581-5602, 2015
- 10Silver (Ag, nanoAg) as an emerging contaminant in the Gironde estuary: scientific assessments and risk governance, Salles D. et al, ERS, 12: 317-323, July/August 2013
- 11“Fate of nanomaterials in the water ecosystem” in Impact de nanoparticules de TiO2 et de nanotubes de carbone sur les végétaux, thesis, Camille Larue, 2011
- 12Aggregation and Dissolution of 4 nm ZnO Nanoparticles in Aqueous Environments: Influence of pH, Ionic Strength, Size, and Adsorption of Humic Acid, Bian SW et al, Langmuir, 27 (10), pp 6059-6068, 2011
- 13Aggregation and Dissolution of 4 nm ZnO Nanoparticles in Aqueous Environments: Influence of pH, Ionic Strength, Size, and Adsorption of Humic Acid, Bian SW et al, Langmuir, 27 (10), pp 6059-6068, 2011
- 14A research team in the USA has shown the accumulation of single-walled carbon nanotubes in wetland sediments: Fate of single walled carbon nanotubes in wetland ecosystems, Schierz A et al, Environ. Sci: Nano, 2014 (see also associated press release: Nanoparticles accumulate quickly in wetlands, Science Daily, October 1, 2014)
- 15See in particular Transport of nanoparticulate TiO2 UV-filters through a saturated sand column at environmentally relevant concentrations, Motellier D et al, Science of the Total Environment, 811, 152408, March 2022 and the references cited in the report Toxicity and ecotoxicity of carbon nanotubes, ANSES, February 2011
- 16of organic nanoparticles in the aqueous environment, Kummerer K et al, Chemosphere, 82(10):1387-92, 2011
- 17Cf. Les nanoparticules : quels risques en Seine ?, Yann Sivry et al., communication aux 22èmes Journées Scientifiques de l’Environnement – Reconquête des environnement urbains : les défis du 21ème siècle, février 2011
- 18Cf. A review of the properties and processes determining the fate of engineered nanomaterials in the aquatic environment, Peijnenburg W et al, Critical Reviews in Environmental Science and Technology, 45(19): 2084-2134, 2015
- 19Background note – “Pollution” workshop – “Reducing pollution and impacts on biodiversity” – April 2010 (for the French Biodiversity Conference in May 2010)
- 20Uptake and retention of metallic nanoparticles in the Mediterranean mussel (Mytilus galloprovincialis), Aquatic Toxicology, May 2013,
- 21See for example Evidence for Biomagnification of Gold Nanoparticles within a Terrestrial Food Chain, Judy. J et al., About. Sci. Technol., 45 (2), 776-781 (2011); Food Chain Transport of Nanoparticles Affects Behaviour and Fat Metabolism in Fish, Cedervall T. et al, PLoS ONE, 7(2): e32254 (2012)
- 22Prise en compte de l’évolution de l’état d’agglomération dans l’étude de l’écotoxicité des nanoparticules, Nicolas Manier, Rapport scientifique 2013-2014, INERIS, November 2014, p.16
- 23See for example:
– Comparative evaluation on the toxic effect of silver (Ag) and zinc oxide (ZnO) nanoparticles on different trophic levels in aquatic ecosystems: A review, Sibiya A et al, Journal of applied toxicology, 2022 Nanomaterials interact with agricultural pesticides, increasing toxicity to fish, The Organic Center, February 2015 (lay summary of scientific article Ecotoxicological effects of carbofuran and oxidised multiwalled carbon nanotubes on the freshwater fish Nile tilapia: Nanotubes enhance pesticide ecotoxicity, Ecotoxicology and Environmental Safety, 111: 131-137, January 2015)
– Fate of single walled carbon nanotubes in wetland ecosystems, Schierz A et al, Environ. Sci.: Nano, 2014 (and associated press release: Nanoparticles accumulate quickly in wetlands: Aquatic food chains might be harmed by molecules ‘piggybacking’ on carbon nanoparticles, Science Daily, October 1, 2014
– Spatial distribution, electron microscopy analysis of titanium and its correlation to heavy metals: Occurrence and sources of titanium nanomaterials in surface sediments from Xiamen Bay, China, Luo Z et al, J. About. Monit., 13, 1046-1052, 2011: this study of sediments from Xiamen Bay in China showed that these sediments contained up to 2.74 g Ti/kg, largely in the form of 300 nm agglomerates composed of nanoparticles of about 50 nanometers. The distribution of titanium in sediments is positively correlated to that of elements such as lead or zinc, which is consistent with the adsorption of pollutants on the surface of nanoparticles. - 24Cf. Nanomaterials in sunscreens and boats leave marine life vulnerable, UC Davis News, May 12, 2015 (press release); Copper oxide and zinc oxide nanomaterials act as inhibitors of multidrug resistance transport in sea urchin embryos: Their Role as Chemosensitizers, WU B et al., About. Sci. Technol., 49 (9): 5760-5770, April 2015
- 25Cf. Titanium dioxide nanoparticles enhance mortality of fish exposed to bacterial pathogens, Jovanovic B et al, Environmental Pollution, 203: 153-164, August 2015
- 26See in particular
– Ecotoxicological Effects of Transformed Silver and Titanium Dioxide Nanoparticles in the Effluent from a Lab-Scale Wastewater Treatment System, Georgantzopoulou A et al., About. Sci. Technol., 52, 16, 9431-9441, 2018
– The true effects of nanoparticles in their environment, CORDIS, March 2018 : “Most synthetic nanomaterials emitted into the environment will sooner or later arrive in our oceans and seas. The SOS-Nano project designed tests to predict their toxicity to the marine environment. The researchers used an ingenious natural in vivo water exposure system to test the effects of metal oxide nanoparticles: zinc oxide (ZnO) and manganese dioxide (MnO2). Oyster larvae suffered from a high level of toxicity occasioned by ZnO, in contrast, MnO2 NPs were not toxic in all exposure scenarios.” - 27What interactions between nanoparticles and other environmental contaminants, Camille Larue, Bulletin de veille scientifique (BVS), Anses, December 2014



